Question
Question: The following are two reaction-schemes involving magnesium. Find Compounds A to J based on the obser...
The following are two reaction-schemes involving magnesium. Find Compounds A to J based on the observations given are
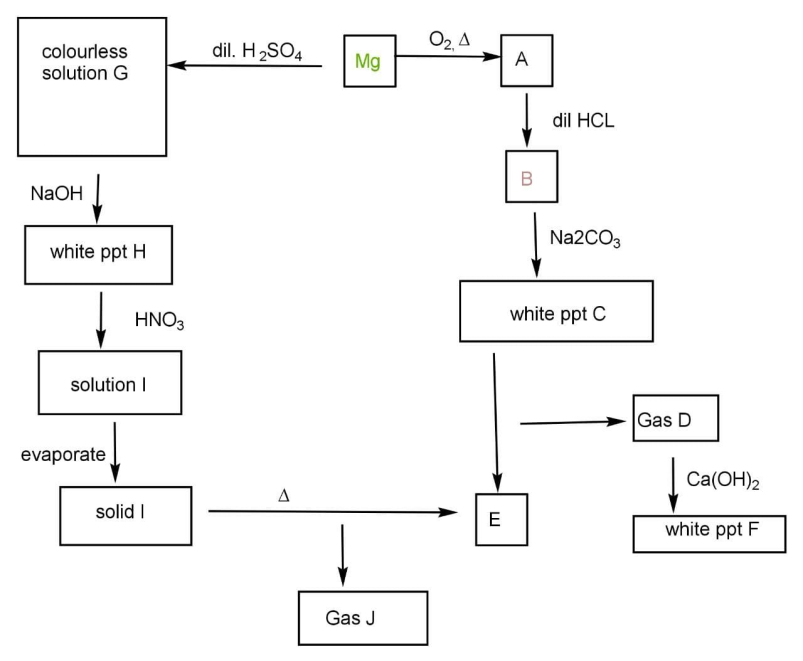
A.A: MgO
B.D: CO2
C.F: CaCO3
D.J: NO2
D.All of these correct.
Solution
Magnesium oxidised in the presence of oxygen. In the presence of acid displacement reaction occurs. The gas D released plays an important role in the respiration process. Magnesium hydroxide is white in colour.
Complete step by step answer:
It is a two way reaction that is the reaction the magnesium is treated with two different reagents and they ultimately form the same products.
First when magnesium is reacted with oxygen then magnesium oxide is formed. It is a very common reaction of burning of magnesium ribbon as the reaction occurs as:
2Mg+O→2MgO
When magnesium oxide is treated with dilute hydrochloric acid then the displacement reaction occurs and oxygen is replaced by chlorine. The formation of magnesium chloride occurs:
MgO+2HCl→MgCl2
Again magnesium chloride reacts with sodium carbonate white colour precipitate of magnesium carbonate. The white precipitate C of magnesium carbonate is:
MgCl2+Na2CO3→2NaCl+MgCO3
When we heat carbonates and carbon dioxide gas is released. So heating magnesium carbonate will give us magnesium oxide the compound E and carbon dioxide gas D. the reaction occurs as:
MgCO3Δ MgO+CO2
When carbon dioxide reacts with calcium hydroxide, it forms calcium carbonate, CaCO3 that id compound F and we all know the colour of calcium carbonate is white.
The product E formed is magnesium oxide. So this side of reaction we have done, now we move towards the left hand side:
Magnesium reacts with sulphuric acid to form magnesium sulphate and hydrogen gas is released.
Mg+H2→4MgSO4+H2
The solution of magnesium sulphate is colourless. When we react with magnesium sulphate with Sodium hydroxide it forms the compound H that is magnesium hydroxide which is white in colour.
Magnesium hydroxide when treated with nitric acid forms magnesium nitrate:
Mg(OH)2+2HNO3→Mg(NO3)2+2H2O
When the solution of magnesium nitrate is heated we get solid magnesium nitrate.
Heating solid magnesium nitrate will give us nitrogen dioxide gas and magnesium oxide which is the compound E. The gas J is nitrogen dioxide:
Mg(NO3)2ΔMgO+NO2
Hence, cycle completes and all the given options are correct.
Hence, the correct option is E.
Note:
Magnesium belongs to group number 2 of modern periodic table. Its atomic number is 12 and mass number is 24. It is a second period element. It is also known as alkaline earth metal because its oxide and hydroxide is basic in nature.
