Question
Question: The five d-orbitals are designated as \({{d}_{xy}},{{d}_{yz}},{{d}_{zx}},{{d}_{{{x}^{2}}-{{y}^{2}}}}...
The five d-orbitals are designated as dxy,dyz,dzx,dx2−y2,dz2 . Choose the correct statement from the given options.
[A] The shape of the first three orbits is similar but that of the fourth and fifth orbitals are different.
[B] The shape of all the five d-orbitals are similar.
[C] The shape of the first four orbitals are similar and that of the fifth orbital is different.
[D] The shapes of all the five d-orbitals are different.
Solution
d-orbitals have a double dumbbell shape. The subscripts in the designation of the d-orbitals depict the orientation of the orbitals along the axes. Their shape is often known as clover like.
Complete step by step answer:
We know that the d sublevel has 5 energy orbitals. The maximum number of electrons in one orbital is 2 and thus the 5 orbitals can fit in maximum 10 electrons.
The five d-orbitals are designated as dxy,dyz,dzx,dx2−y2,dz2 depending upon the plane they are lying upon. The d-orbitals have a double dumbbell shape and thus they are named differently depending upon how the double dumbbell is oriented in the axes.
Now, let us discuss their shapes and their orientation-
- The orbital lying in the xy-plane is designated as dxy.
- The orbital lying in the yz-plane is designated as dyz.
- The orbital lying in the xy-plane is designated as dzx.
- The dx2−y2 orbital lies on the x-y axes.
- The dz2 orbital has a single dumbbell shape and it lies along the z-axis. There is a ring of negative charge around it along the xy-plane.
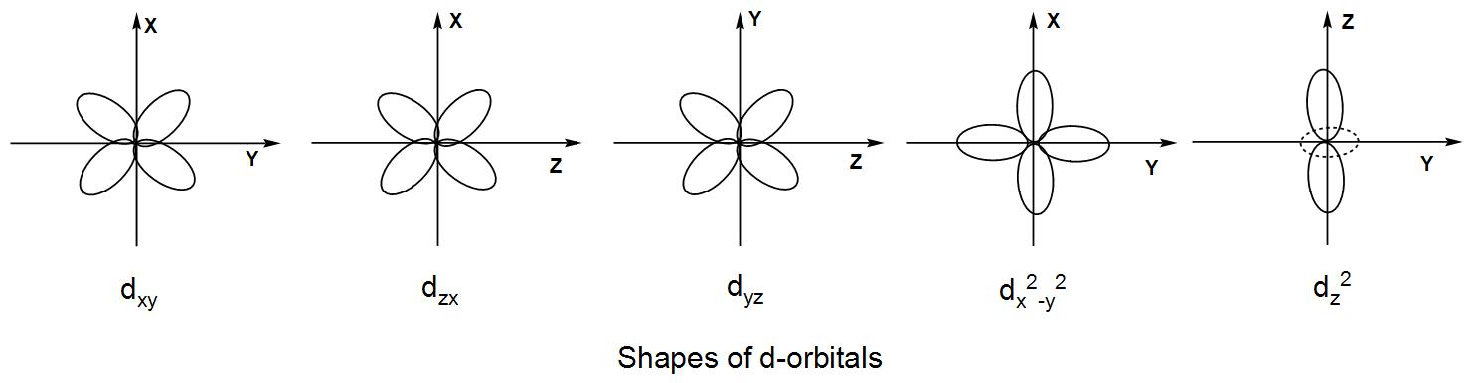
The four d-orbitals have similar double dumbbell shape just in the fourth orbital the structure is oriented along the axes instead of a plane and the fifth orbital has a single dumbbell shape with a ring around it and it is often called a donut shaped orbital.
We can understand from the above discussion that all the orbitals have different orientations but the four d-orbitals have a similar shape and the fifth one has a different shape compared to the others.
So, the correct answer is “Option C”.
Note: The orbitals differ in shape due to having different probabilities of finding electrons around the nucleus. The point where there is no possibility of finding a nucleus is known as a node. In d-orbitals, there are 2 nodal planes. This is due to their double dumbbell shape.
