Question
Question: The final product of the given reaction is, 
Solution
Here, first we have to find out the product C of the second reaction. We know that an ester when reacts with sodium hydroxide forms carboxylate ion and alcohol. Further reaction with acid produces the carboxylic acid.
Complete step by step answer:
Let’s try to find the product C in the second reaction. The given reactant Ph−COOCH3 is an ester. And we know the reaction of ester with sodium hydroxide produces carboxylate ion and alcohol. Then, the reaction of carboxylate ion with acid gives carboxylic acid. The reaction can be shown as follows:
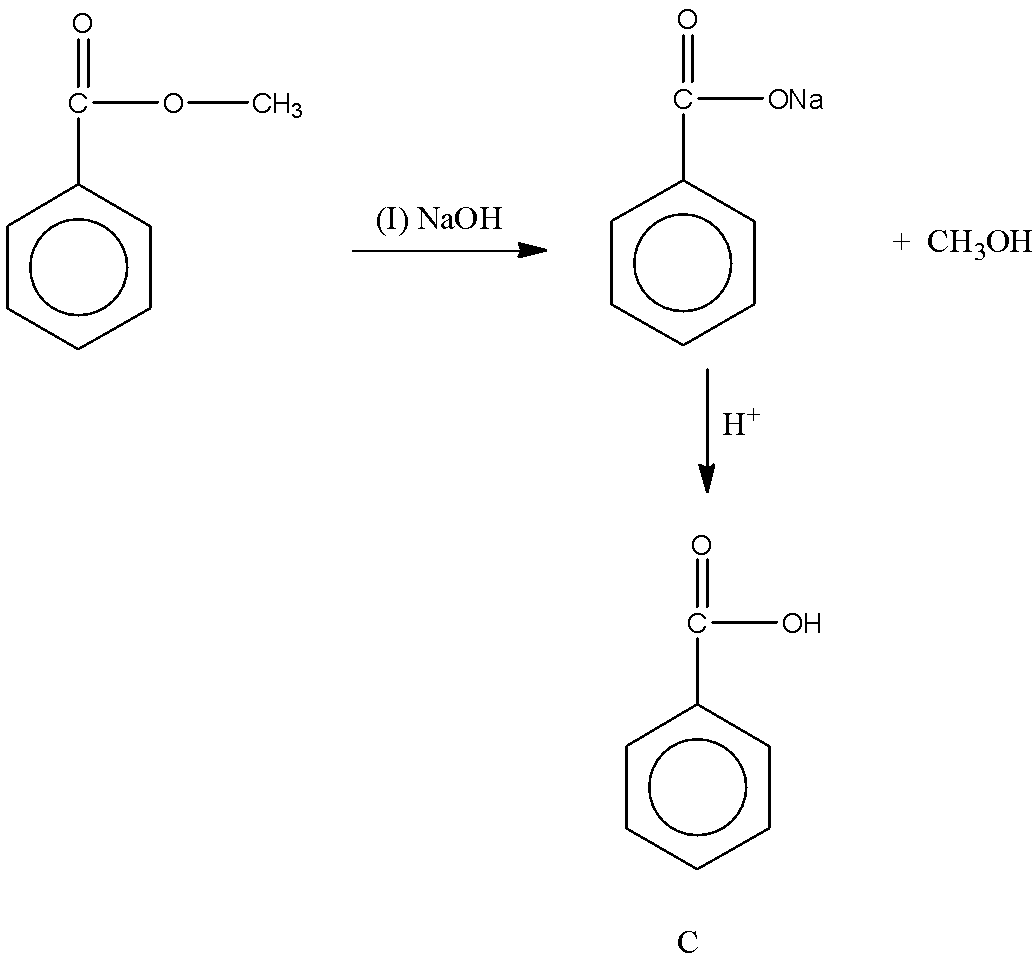
So, we obtain the product C.
Now we have to find the final product of the first reaction.
Here, Ph−COOCH3 reacts with water to produce an acid and an alcohol in presence of an acid.
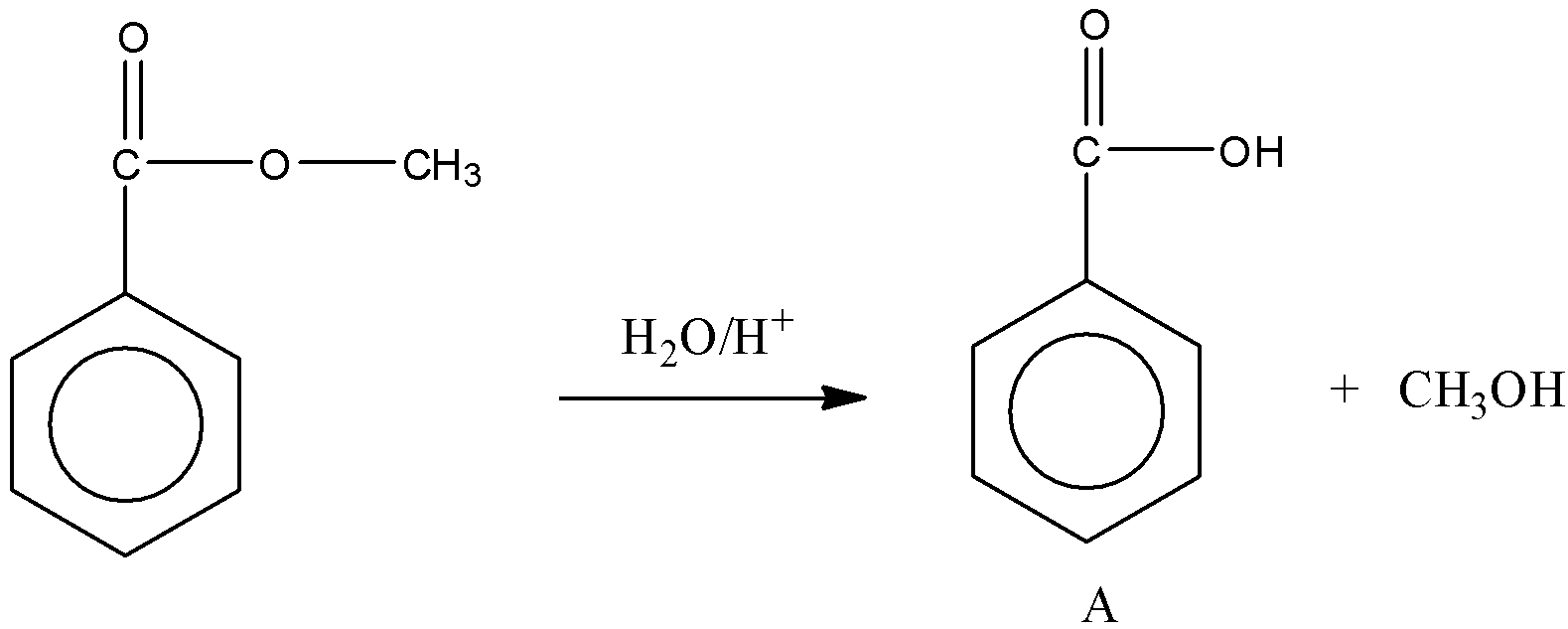
So, we obtain the product A.
Then, A reacts with SOCl2. We know that when carboxylic acid reacts with SOCl2, the product formed is acid chloride.
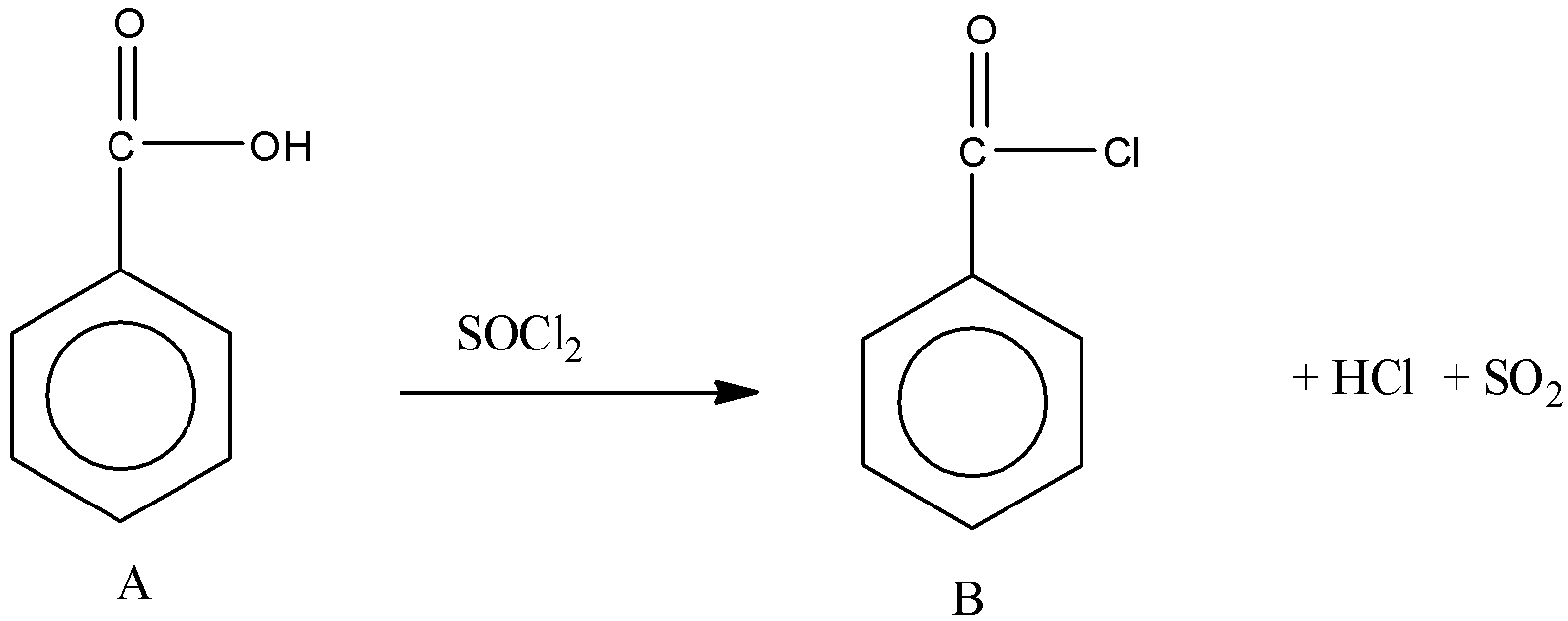
Now, the acid chloride undergoes reaction with C (second reaction). The product C formed is a carboxylic acid. So, the reaction is between acid chloride and carboxylic acid. The reaction between carboxylic acid and acid chloride forms acid anhydride. So, the reaction is,
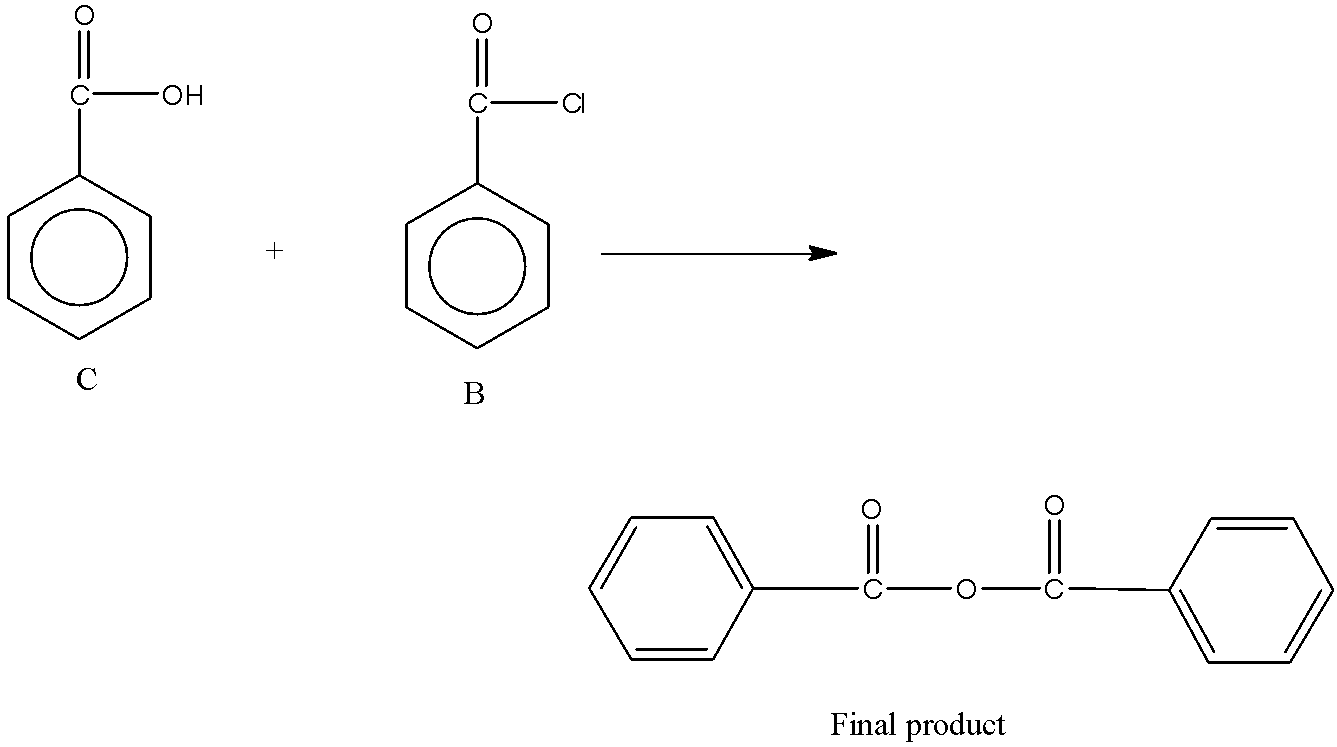
Note: It is to be noted that the reaction of ester with sodium hydroxide is also known by the name of saponification reaction. In this reaction, fat, lipid or oil is transformed into alcohol and soap by heating it in presence of sodium hydroxide. The saponification value indicates the quantity of base (NaOH) needed to saponify a sample of fat.
