Question
Question: The figures given below show different processes (relating pressure P and volume V) for a given amou...
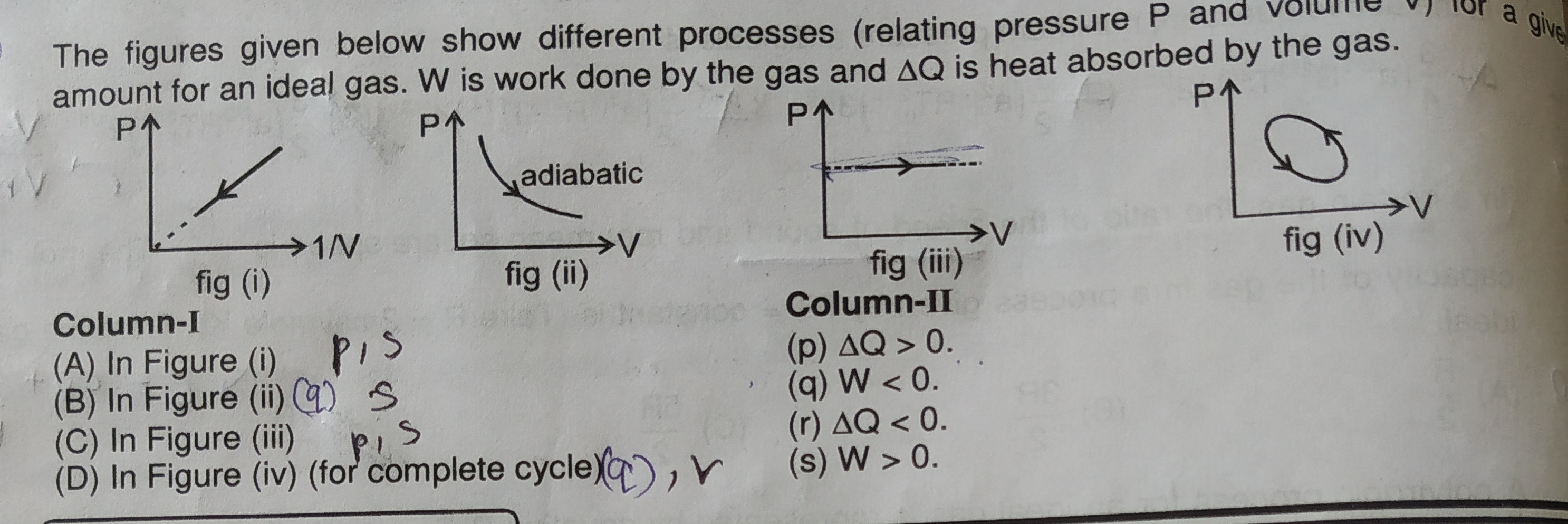
The figures given below show different processes (relating pressure P and volume V) for a given amount for an ideal gas. W is work done by the gas and ΔQ is heat absorbed by the gas.
Column-I
(A) In Figure (i) p,s (B) In Figure (ii) (q), s (C) In Figure (iii) p,s (D) In Figure (iv) (for complete cycle)(q),r
ΔQ > 0.
W < 0.
ΔQ < 0.
W > 0.
C, D
Solution
The problem requires us to analyze four different thermodynamic processes for an ideal gas and match them with the given conditions for work done (W) by the gas and heat absorbed (ΔQ) by the gas.
We will use the following principles:
-
Work done (W) by the gas:
- W=∫PdV.
- If the volume (V) increases (expansion), W>0.
- If the volume (V) decreases (compression), W<0.
- On a P-V diagram, W is the area under the curve.
- For a cyclic process, W is the area enclosed by the loop. If the cycle is traversed clockwise, W>0. If counter-clockwise, W<0.
-
First Law of Thermodynamics:
- ΔQ=ΔU+W, where ΔU is the change in internal energy.
- For an ideal gas, ΔU=nCvΔT.
- If temperature (T) increases, ΔU>0. If T decreases, ΔU<0. If T is constant (isothermal), ΔU=0.
- For a cyclic process, ΔU=0 as the system returns to its initial state. Therefore, ΔQ=W.
Let's analyze each figure:
Figure (i): P vs 1/V graph
- The graph shows a straight line passing through the origin, implying P∝V1, or PV=constant.
- For an ideal gas, PV=nRT. Since PV is constant, the temperature (T) must be constant. This is an isothermal process.
- The arrow indicates that P increases and 1/V increases. If 1/V increases, then V decreases. This is an isothermal compression.
- Work done (W): Since the volume decreases (dV<0), work is done on the gas, so work done by the gas is negative. W<0. (Matches (q))
- Heat absorbed (ΔQ): For an isothermal process, ΔU=0. According to the First Law of Thermodynamics, ΔQ=ΔU+W=0+W=W. Since W<0, then ΔQ<0. (Matches (r))
- So, for Figure (i), the correct matches are (q), (r).
Figure (ii): P vs V graph (adiabatic expansion)
- The graph is explicitly labeled "adiabatic".
- The arrow indicates that volume (V) increases and pressure (P) decreases. This is an adiabatic expansion.
- Work done (W): Since the volume increases (dV>0), work is done by the gas. W>0. (Matches (s))
- Heat absorbed (ΔQ): By definition, for an adiabatic process, there is no heat exchange with the surroundings. So, ΔQ=0.
- So, for Figure (ii), the correct match is (s). (Note: ΔQ=0 means neither (p) nor (r) applies).
Figure (iii): P vs V graph (isobaric expansion)
- The graph shows a horizontal line, meaning pressure (P) is constant. This is an isobaric process.
- The arrow indicates that volume (V) increases while P remains constant. This is an isobaric expansion.
- Work done (W): Since the volume increases (dV>0), work is done by the gas. W>0. (Matches (s))
- Heat absorbed (ΔQ): For an ideal gas undergoing isobaric expansion, P is constant and V increases. From PV=nRT, if P is constant and V increases, then temperature (T) must increase (ΔT>0).
- Change in internal energy ΔU=nCvΔT. Since ΔT>0, ΔU>0.
- From the First Law of Thermodynamics, ΔQ=ΔU+W. Since both ΔU>0 and W>0, then ΔQ>0. (Matches (p))
- So, for Figure (iii), the correct matches are (p), (s).
Figure (iv): P vs V graph (cyclic process)
- The graph shows a closed loop, indicating a cyclic process.
- The arrows indicate a counter-clockwise direction.
- Work done (W): For a cyclic process on a P-V diagram, the work done is the area enclosed by the loop. For a counter-clockwise cycle, work is done on the gas, so W<0. (Matches (q))
- Heat absorbed (ΔQ): For any cyclic process, the system returns to its initial state, so the change in internal energy (ΔU) is zero.
- From the First Law of Thermodynamics, ΔQ=ΔU+W. Since ΔU=0, then ΔQ=W.
- Since W<0 for this counter-clockwise cycle, then ΔQ<0. (Matches (r))
- So, for Figure (iv), the correct matches are (q), (r).
Now, let's compare our analysis with the given options in Column-I:
- (A) In Figure (i) p,s: Our analysis: (q), (r). So (A) is incorrect.
- (B) In Figure (ii) (q), s: Our analysis: (s) and ΔQ=0. The option lists (q) W<0 which contradicts (s) W>0. Since Figure (ii) is an expansion, W>0 is correct, making (q) incorrect. So (B) is incorrect.
- (C) In Figure (iii) p,s: Our analysis: (p), (s). This matches the option perfectly. So (C) is correct.
- (D) In Figure (iv) (for complete cycle)(q),r: Our analysis: (q), (r). This matches the option perfectly. So (D) is correct.
Thus, both options (C) and (D) are correct.
