Question
Question: The figure shows two processes A and B for a gas. If \[\Delta {Q_A}\]and \[\Delta {Q_B}\]are the amo...
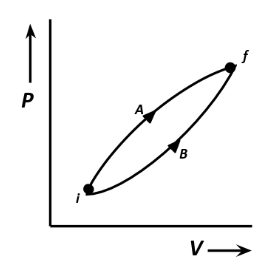
The figure shows two processes A and B for a gas. If ΔQAand ΔQBare the amount of heat absorbed by the system in the two cases and ΔUA and ΔUBare the changes in the internal energies, respectively, then:
A. ΔQA=ΔQB;ΔUA=ΔUB
B. ΔQA>ΔQB;ΔUA=ΔUB
C. ΔQA>ΔQB;ΔUA>ΔUB
D. ΔQA<ΔQB;ΔUA<ΔUB
Solution
Two systems are given such as A and B for a gas. From the given diagram, the final state and the initial state are the same for both the systems. The amount of heat absorbed by the systems depends on the work done by the systems.
Complete step by step solution: The term internal energy U implied to the energy within the system. It consists of Kinetic and potential energies. The change in internal energy of the system depends on the initial and the final states (such as pressure, volume and temperature) of the system.
From the given diagram, the internal energies for the systems A and B are the same. Therefore ΔUA=ΔUB
The first law of thermodynamics states that a system absorbs heat ΔQ and as a result the internal energy of the system changes by ΔU and the system does a work ΔW.
ΔQ=ΔU+ΔW
where, ΔQ is the heat absorbed by the system
ΔU is the change in internal energy of the system
ΔW is the work done by the system
Thus, the amount of heat absorbed by the systems depends on the work done by the systems.
The work done by the systems is defined as the area under the curve in the graphs of the systems. From the graph, the area under the curve of system A is greater than the area under curve of system B.
Thus the work done by system A is greater than that of system B. Therefore, the amount of heat absorbed by system A will be greater than system B. Thus, ΔQA>ΔQB
Hence, option (B) is the correct answer.
Note: It is not possible to determine the absolute value of the internal energy of the system. Only the changes in internal energies can be measured. The change in internal energy of the system depends on the initial and the final states of the system and is independent of the manner in which the changes are brought about.
