Question
Question: The figure above shows the electron energy levels, referred to the ground state (the lowest possible...
The figure above shows the electron energy levels, referred to the ground state (the lowest possible energy) as zero, for four different isolated atoms. Which atom can produce radiation of the shortest wavelength when atoms in the ground state are bombarded with electrons of energy W?
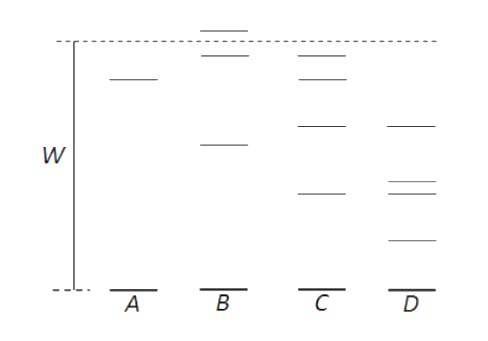
(a). A
(b). B
(c). C
(d). D
Solution
For energy of energy states in atoms, it always depends on the wavelength and the frequency. Obtain the relation between energy and frequency or wavelength. Energy is directly proportional to frequency while inversely proportional to the wavelength.
Complete step by step answer:
The figure above shows the electron energy levels, referred to the ground state (the lowest possible energy) as zero, for five different isolated atoms. When atoms in the ground state are bombarded with electrons of energy W, some atoms in the ground state acquire that much energy to get excited to the highest energy level. That difference in energy level is given by the formula,
ΔE=λhc
Where, ΔE is the difference in energy levels, h is the Planck’s constant, c is the speed of light and λ is the respective wavelength.
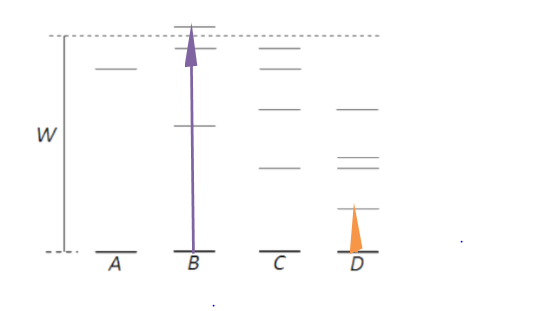
According to the above equation, energy difference is only dependent on wavelength because h and c are constants.
If atoms acquire less energy then, the atoms get excited to the lower energy state and vice versa. That atom can produce radiation which has the shortest wavelength when atoms in the ground state are bombarded with electrons of energy W.
In the above figure atom B has maximum energy difference and hence it can produce radiation of shortest wavelength. Atom D has a minimum energy difference, so it can produce radiation of the largest wavelength.
Note:
Sometimes ΔE is also written as only E. Above equation it is for the energy difference of energy states and not only the value of energy. Energy has the unit of joule as well as electron-volt. When energy is given to the atom the electrons in it jumps to higher energy levels. When the electrons come to a stable energy state, then also we get emission of radiation.
