Question
Question: The expansion of a mass \(m\) of an ideal gas at a constant pressure \(P\) is shown by the line H. T...
The expansion of a mass m of an ideal gas at a constant pressure P is shown by the line H. The expansion of a mass 2m of the same gas at a pressure 2P is shown by.
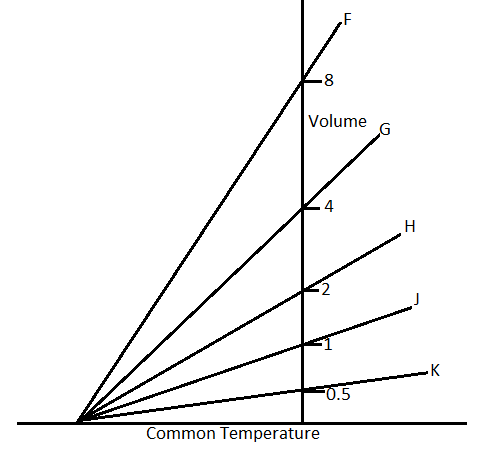
(A) Line F
(B) Line G
(C) Line H
(D) Line J or line K
Solution
Hint To solve this question, we need to use the ideal gas law PV=nRT to find the expression for the volume. Now by putting the value of mass and pressure in the first case we get the expression for volume V1 and similarly for the second case, we find the expression of V2 and the write it in terms of V1. From the graph we can obtain the value of V1 and hence the value of V2. The line corresponding to the value of V2 in the graph gives the answer.
Formula Used
In the solution of this question we use the formulae,
PV=nRT
where P is the pressure of the gas, V is the volume of the gas, n is the number of moles, R is the universal gas constant and T is the temperature of the gas.
n=Mm where m is the mass and M is the molar mass.
Complete step by step answer:
At any constant temperature T from the gas equation, we have
PV=constant which is given by nRT.
So in the first case, we are given the mass of an ideal gas is m. Hence, the number of moles for m mass is given by, n1=Mm where M is constant for any gas and the pressure is P1=P.
So from the gas equation
P1V1=n1RT
⇒V1=P1n1RT
Now by substituting the values of the P1 and n1 in the equation, we get,
V1=PMmRT
For the second case, the mass is given by 2m and the number of moles is, n2=M2m and the pressure is given by, P2=2P.
Therefore from the gas equation,
P2V2=n2RT
⇒V2=P2n2RT
So by substituting the values of n2 and P2 we get,
V2=2PM2mRT
⇒V2=4PMmRT
As we know have already calculated, V1=PMmRT so by substituting this value in the equation of V2 we get,
V2=4V1
⇒V1V2=4
In the question, it is said that the volume in the first case, V1 is given by the line H. So from the graph, the volume V1 is 2.
By substituting this value in the equation of V2 we get,
V2=4V1
Putting the value of V1 we get,
V2=4×2=8
According to the graph, the line F cuts the volume for V2=8.
Therefore, the answer will be line F, which is option A.
Note We can also solve this problem by taking the slope of the curve. Since
PV=nRT
⇒PV∝MT where M is constant
So we can write,
⇒V∝PMT
So in the Volume-Temperature graph as in the question PM is the slope.
In the first case, the slope is given as 2.
For the second case, mass and pressure are 2M and 2P, respectively.
So, we get,
V∝2P2M∝4PM.
As PM=2, for the second case, the slope is 4×2=8.
The answer is 8.
