Question
Chemistry Question on Equilibrium Constant
The equilibrium constant for the equilibrium PCl5(g)⇌PCl3(g)+Cl2(g) at a particular temperature is 2×10−2molL−1. The number of moles of PCl5 that must be taken in a one litre flask at the same temperature to obtain a concentration of 0.20 mole of chlorine at equilibrium is
A
2.2
B
2
C
1.8
D
0.2
Answer
2.2
Explanation
Solution
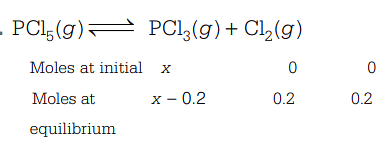 So, equilibrium constant, K=[PCl5][PCl3][Cl2] [given, K=2×10−2mol/L] ∴2×10−2=x−0.20.2×0.2 ⇒x=2.2 moles
So, equilibrium constant, K=[PCl5][PCl3][Cl2] [given, K=2×10−2mol/L] ∴2×10−2=x−0.20.2×0.2 ⇒x=2.2 moles
