Question
Question: The equation for Freundlich adsorption isotherm is: A. \[\dfrac{x}{m} = K{p^{1/n}}\] B. \[x = m...
The equation for Freundlich adsorption isotherm is:
A. mx=Kp1/n
B. x=mKp1/n
C. mx=Kp−n
D. All of these
Solution
From the name itself, it becomes clear that Freundlich adsorption isotherm is a curve that expresses the variation in the amount of gas adsorbed by the adsorbent with the temperature at the constant pressure.
Complete step-by-step answer:
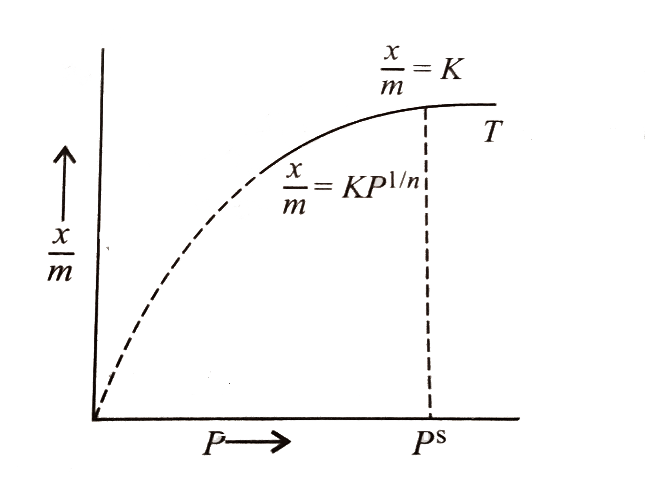
The Freundlich adsorption isotherm is an empirical relation between the concentrations of a solute on the surface of an adsorbent to the concentration of the solute present in the liquid. Freundlich equation is an expression which represents the isothermal variation of adsorption of a gas adsorbed on a unit mass solid adsorbent with pressure. Adsorption is an attractive interaction between atoms, molecules or ions of a gas or liquid to a surface or deposition of molecular species on a surface called adsorbent.
Its mathematical expression can be analysed as:
mx=Kp1/n(n> 1)
Where, x is the mass of the adsorbent, k and n are constants that depend on the nature of adsorbent and the gas at a specific temperature .Adsorption factors consists of temperature, pore-volume, degree of saturation, molecular sieve, type of adsorbent, and surface area.
__
Hence, the correct option is (A).
Note: One might get confused between adsorption and absorption. They seem similar but are totally different as absorption is a bulk process in which the particles go into the bulk of the solvent while adsorption is a surface phenomenon in which the particles remain stuck to the surface of the solvent.
