Question
Question: The enthalpy of combustion of propane (\({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{8}}}\) ) gas in t...
The enthalpy of combustion of propane (C3H8 ) gas in terms of given data is:
Bond energy (kJ/mol)
| εC - H | εO = O | εC = O | εO - H | εC - C |
|---|---|---|---|---|
| +x1 | +x2 | +x3 | +x4 | +x5 |
Resonance energy of CO2 is −zkJ/mol and ΔHvaprization [H2O(l)] is y (kJ/mol)
A. 8x1 +2x5 +5x2−6x3−8x4−4y−3z
B. 6x1 +x5 +5x2−3x3−4x4−4y−3z
C. 8x1 +2x5 +5x2−6x3−8x4−y−z
D. 8x1 +x5 +5x2−6x3−8x4−4y+3z
Solution
Write the balanced reaction for the combustion of propane. Determine the number of each type of bond present in reactants and products. Calculate the enthalpy combustion of propane using bond energy of reactants, bond energy of products, resonance energy of CO2 and heat of vaporization of water.
Complete Step by step answer:
A combustion reaction is a type of reaction where hydrocarbon reacts with oxygen gas and gives carbon dioxide gas and water vapor.
So, the unbalanced combustion reaction of propane is:
C3H8 + O2 → CO2 + H2O
Now, the balanced reaction for the combustion of propane is:
C3H8 + 5O2 → 3CO2 + 4H2O
Now, using the given data calculates the bond energy of reactant ( BEr) and bond energy of product (BEP) as follows:
Structure of propane is:
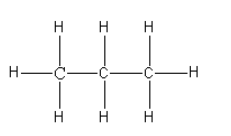
So, in propane, there are 8 C-H bonds and 2C-C bonds present.
Structure of O2is :
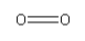
In oxygen gas, there is 1O = O double bond present.
Structure of CO2 is :
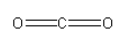
In CO2 there are 2 C = O bonds present.
Structure of H2O is:
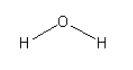
In water there are 2 O-H bonds present.
Now calculate the bond energy of reactant and product using balance combustion reaction of propane as follows:
C3H8 + 5O2 → 3CO2 + 4H2O
Here, 1 mole of propane is reacting with 5 moles of O2 and gives 3 moles of CO2 and 4 moles ofH2O .
So, the bond energy of reactant (BEr) is as follows:
BEr=2C - C + 8 C - H + 5O = O
Now, substitute the bond energy +x1 for C - H, +x5for C - C and +x2 for O = O.
BEr=2x5 + 8 x1 + 5 x2
Calculate bond energy of reactant (BEP) is as follows:
BEP=6C = O + 8O - H
Now, substitute the bond energy +x3 for C = O and +x4for O - H
BEP=6 x3 + 8x4
Now, calculate the enthalpy combustion of propane as follows:
Hr= BEr−BEP+RE - ΔHvap
RE = resonance energy of CO2=−zkJ/mol
ΔHvap= heat of vaporization of water =y (kJ/mol)
Since there are 3 moles of CO2 and 4 moles ofH2O .
Hr= 2x5 + 8 x1 + 5 x2−(6 x3 + 8x4)−3z−4y
Hr= 2x5 + 8 x1 + 5 x2−6 x3 - 8x4−3z−4y
Thus, the correct option is (A).
Note: It is very important to balance the reaction correctly. Also, count the number of types of bonds present correctly. After that we should take care that all the values should be added thoroughly with the proper corrected sign.
