Question
Question: The enthalpy of combustion of propane \[({C_3}{H_8})\] gas in terms of given data is: bond energy(kJ...
The enthalpy of combustion of propane (C3H8) gas in terms of given data is: bond energy(kJ/mol)
6C−H6O=O6C=O6O−H6C−C \+X1+X2+X3+X4+X5Resonance energy of CO2 is -z kJ/mol and ΔHvaporisation[H2O(l)] is y (kJ/mol)
A. 8x1+2x5+5x26x38x44y−3z
B. 6x1+x5+5x23x34x44y−3z
C. 8x1+2x5+5x26x38x4y−z
D. 8x1+x5+5x26x38x44y+3z
Solution
The enthalpy change in the chemical reactions is due to the bond breaking and making process so as to form new products from the provided reactants. Enthalpy of combustion can be represented as the difference between bond-breaking energies of reactants and bond-breaking energies of products, along with addition of enthalpy of vaporization and subtraction of resonance energy.
Complete step by step solution:
To find the enthalpy of combustion of propane, let us define what is combustion. Combustion is a process in which a combustible substance burns in presence of oxygen to form water and release carbon dioxide gas. These combustible substances are generally hydrocarbons i.e. compounds containing hydrogen and carbon atoms only.
We can write the balanced chemical equation of the chemical reaction i.e. combustion of propane gas as:
C3H8(g)+5O2(g)→3CO2(g)+4H2O(l)
For this equation, we are given the bond energies of all types of bonds present in the compounds taking part in the reaction in terms of variable x. Also, we are provided with resonance energy of carbon dioxide which is the product of this combustion reaction as -z kJ/mol and enthalpy of vaporization of water produced as y kJ/mol.
Now let us look at the type of bonds present in C3H8 by drawing its open structure.
__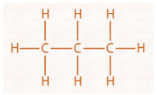
From this structure, we can see that it has two (C−C) bonds and eight (C−H) bonds. Also, we know that one oxygen atom binds with another oxygen atom with a double bond between them (O=O) . since we have five oxygen molecules, we will have five (O=O) bonds. Now, let us look at the structure of carbon dioxide which is (O=C=O) , so we will have six (C=O) bonds as three molecules of carbon dioxide are released. Water molecule has a structure (H−O−H) , so we can have eight bonds of (H−O) as four molecules of water are produced in the reaction.
We can write the enthalpy of combustion in terms of bond-breaking as:
ΔHcombustion=ΔHbond−breaking(reactants)−ΔHbond−breaking(products)
Now, since resonance energy of carbon dioxide will be provided from outside, we will have to add it in the above formula and enthalpy of vaporization has to be subtracted at the same time for water molecules produced as it is the energy released during its production. The modified formula will be:
ΔHcombustion=ΔHbond−breaking(reactants)−ΔHbond−breaking(products)−4y+3z where y is the given enthalpy of vaporization of liquid water and z is the resonance energy of carbon dioxide given to us.
Now let us substitute the bonds of reactant and products in the above formula along with prefixes of their numbers.
ΔHcombustion=[εC−C+εC−H+εO=O]−[εC=O+εH−O]−4y+3z
We are given the variable term for each bond, on substituting them in this formula, we get
ΔHcombustion=8x1+2x5+5x26x38x44y−3z
Hence, the correct option is (A).
Note:
The enthalpy change can be either exothermic or endothermic. It is endothermic when the bonds of the reactants which need to be broken are very strong and require more energy than what is evolved during the formation of new bonds in the product. Whereas it is exothermic if the formation of new bonds liberates more energy than what was used in the initial bond breaking process of reactants.
