Question
Question: The enolic form of acetone contains A. \(9\) sigma bonds, \(1\) pi bond and \(2\) lone pairs of el...
The enolic form of acetone contains
A. 9 sigma bonds, 1 pi bond and 2 lone pairs of electrons
B. 8 sigma bonds, 2 pi bond and 2 lone pairs of electrons
C. 10 sigma bonds, 1 pi bond and 1 lone pairs of electrons
D. 9 sigma bonds, 2 pi bond and 1 lone pairs of electrons
Solution
Count the single bonds for calculating sigma bonds. Count the double bonds for calculating pi bonds. For lone pairs, look for the valence electrons of each atom which are not participating in bond formation.
Complete step-by-step answer:
First let us see what terms we are dealing with in the option. First we have a sigma bond. Sigma bond is type of a covalent bond. It is the strongest type of covalent bond. It is formed when by head on overlapping atomic orbitals. It can be identified as a single bond in a structure of compounds.
Next we have pi bonds. Pi bonds are also types of covalent bonds. It is weaker than a sigma bond. It is formed when two lobes of an orbital in an atom overlap another two lobes of an orbital of another atom. It can be identified as the second bond in a double bond in a structure of compound.
At last we have lone pairs. Lone pair is a pair of electrons in the valence shell that do not participate while forming a bond. They come in picture while discussing the formation of covalent bonds. They can be represented as dots around an atom.
To find sigma bond, pi bonds and lone pairs on acetone, let us look at the structure of acetone,
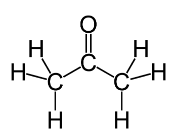
For the number of sigma bonds we can count the number of single bonds in the structure. So there are nine sigma bonds in the structure. For pi bonds we can count the number of double bonds. So there is one pi bond. Carbon and hydrogen’s electrons are occupied. Oxygen has electronic configuration 2,6 and so there are six valence electrons in oxygen and only two of them are used in bonding. So we are left with four valence electrons which makes two electron pairs.
So option A is the correct answer.
Note: A double bond has one sigma bond and one pi bond. One triple bond has one sigma bond and two pi bonds. In a pi bond the orbital paths of combining atoms are parallel so there is less overlapping which results in weakness of pi bond as compared to sigma bond.
