Question
Question: The energy level diagram of the given element is given below. Identify by doing necessary calculatio...
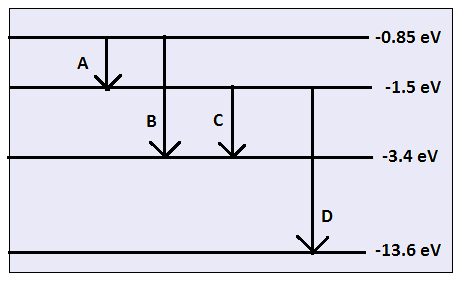
The energy level diagram of the given element is given below. Identify by doing necessary calculation which transition corresponds to the emission of a spectral line of wavelength 102.7 nm.
Solution
The amount of energy associated with the photon of radiation is directly proportional to the frequency of incident light which is expressed as E=hν. It shows that higher the frequency of incident light the higher the energy corresponds to photons.
Complete answer:
As we know the relation between energy of photon and frequency of incident light is E=hν.
Where is defined as a universal constant, known as planck's constant. Value of planck's constant is 6.626×10−34 Js.
As we know frequency is also expressed in terms of light constant and wavelength of radiation.
ν=λc
So, final equation of energy in terms of wavelength becomes-
E=λhc
For element A
E1=−1.5
E2=−0.85
Therefore, difference in energy is
ΔE=(E2−E1)
ΔE=−0.85−(−1.5)
After solving the above equation, we get ΔE=0.65
Now calculate the wavelength of transition
λ=Ehc
λ=0.65×1.6×10−196.626×10−34×3.0×108
After solving this above equation, we get
λ=19.038×10−7m
In terms of nanometer value of wavelength is-
λ=1903.8nm
For element B
E1=−3.4
E2=−0.85
Therefore, difference in energy is
ΔE=(E2−E1)
ΔE=−0.85−(−3.4)
After solving the above equation, we get ΔE=2.55
Now calculate the wavelength of transition
λ=Ehc
λ=2.55×1.6×10−196.626×10−34×3.0×108
After solving this above equation, we get
λ=4.8529×10−7m
In terms of nanometer value of wavelength is-
λ=485.2nm
For element C
E1=−3.4
E2=−1.5
Therefore, difference in energy is
ΔE=(E2−E1)
ΔE=−1.5−(−3.4)
After solving the above equation, we get ΔE=1.9
Now calculate the wavelength of transition
λ=Ehc
λ=1.9×1.6×10−196.626×10−34×3.0×108
After solving this above equation, we get
λ=6.5131×10−7m
In terms of nanometer value of wavelength is-
λ=651nm
For element D
E1=−13.6
E2=−1.5
Therefore, difference in energy is
ΔE=(E2−E1)
ΔE=−1.5−(−13.6)
After solving the above equation, we get ΔE=12.1
Now calculate the wavelength of transition
λ=Ehc
λ=12.1×1.6×10−196.626×10−34×3.0×108
After solving this above equation, we get
λ=1.0227×10−7m
In terms of nanometer value of wavelength is-
λ=102.27nm
Hence, from the above calculation transition of element D from −1.5 ev to −13.6 ev corresponds with the emission of wavelength of 102.27nm.
Note:
Make sure to convert the wavelength of the radiation into nanometers and convert the unit of ΔE from volts to electron-volts by multiplying it by 1.6×10−19.
