Question
Question: The energy diagram of a reaction \(P+Q\to R+S\) is given. What are A and B in the graph? ...
The energy diagram of a reaction P+Q→R+S is given. What are A and B in the graph?
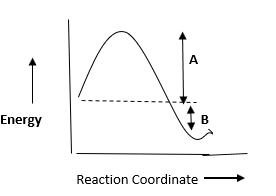
A.A→ activation energy, B→ heat of reaction
B.A→ threshold energy, B→ heat of reaction
C.A→ heat of energy, B→ activation reaction
D.A→ potential energy, B→energy of reaction
Solution
Activation energy is the difference in energy of transition complex and reactants. Heat of reaction is the energy difference between products and the reactants. Potential energy is energy that is stored or conserved in substance.
Complete answer:
Activation Energy:
-Activation energy (Ea) is defined as the minimum amount of extra energy required by a reacting molecule to get converted into a product.
-It can also be described as the minimum amount of energy needed to activate or energize molecules or atoms so that they can undergo a chemical reaction or transformation.
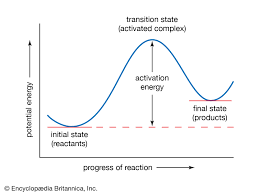
Hence, in the given diagram, A denotes activation energy.
Heat of reaction:
-The heat of reaction is given by:
ΔH=Eaf−Eab
Where,
ΔH = heat of reaction
Eaf = forward activation energy
Eab = backward activation energy
Let's suppose O is the highest point of the curve then RO is forward activation energy and PO is backward activation energy and the difference of these two will give the heat of reaction, [Indicated in the below figure]
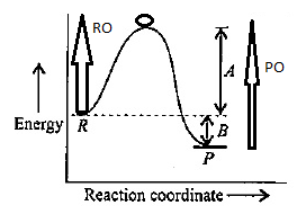
Hence, B in the graph denotes heat of reaction.
So, the correct option is (A) A→ activation energy, B→ heat of reaction.
Note:
Activation energy of a reaction can be altered by addition of a substance called catalyst. Catalyst is a chemical substance which when added into the reaction increases the rate of reaction without itself getting consumed in the reaction. Catalyst increases the rate of reaction by decreasing the activation energy of a reaction.
