Question
Question: The end product 'W' is: ...
The end product 'W' is:
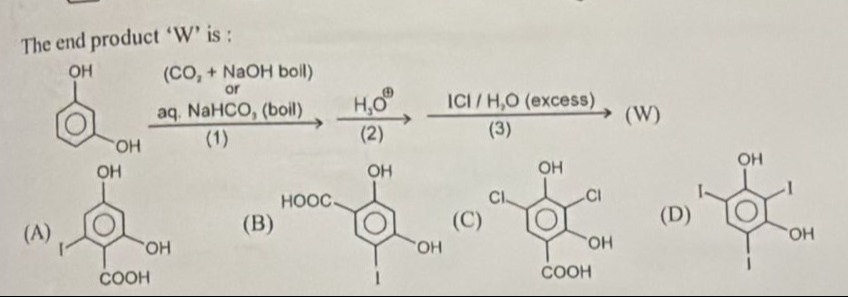
\includegraphics[width=\linewidth]{A}
\includegraphics[width=\linewidth]{B}
\includegraphics[width=\linewidth]{C}
\includegraphics[width=\linewidth]{D}
A
Solution
The starting material is resorcinol (1,3-dihydroxybenzene).
Step 1: Resorcinol undergoes Kolbe's reaction with CO2 and base (NaOH or NaHCO3). Resorcinol is highly reactive towards electrophilic substitution due to the presence of two activating hydroxyl groups. The carboxylation typically occurs at the activated positions. Based on the options provided, the carboxylation occurs at position 4, relative to the hydroxyl groups at positions 1 and 3. Thus, the product after step 1 and 2 (acidification) is 1,3-dihydroxy-4-benzoic acid.
Step 2: 1,3-dihydroxy-4-benzoic acid is treated with ICl/H2O (excess). ICl is a source of electrophilic iodine (I⊕). Electrophilic substitution will occur on the aromatic ring. The hydroxyl groups at positions 1 and 3 are strongly activating and are ortho, para directing. The carboxyl group at position 4 is deactivating and meta directing. The activating effect of the hydroxyl groups dominates over the deactivating effect of the carboxyl group.
Let's consider the positions available for substitution in 1,3-dihydroxy-4-benzoic acid:
- -OH at position 1 activates positions 2, 4 (substituted), and 6.
- -OH at position 3 activates positions 2, 4 (substituted), and 5.
- -COOH at position 4 directs to positions 2, 3 (substituted), 5, and 6.
The available positions for electrophilic substitution are 2, 5, and 6.
- Position 2 is ortho to both OH groups.
- Position 5 is ortho to OH at 3 and para to OH at 1.
- Position 6 is ortho to OH at 1 and para to OH at 3.
Since excess ICl is used, multiple substitutions are expected at the activated positions. Positions 2, 5, and 6 are all activated. Position 2 is the most activated as it is ortho to both hydroxyl groups. Positions 5 and 6 are also activated. Therefore, substitution will occur at positions 2 and 6, leading to a di-substituted product: 2,6-diiodo-1,3-dihydroxy-4-benzoic acid.
