Question
Question: The end product ‘W’ in the following sequence of reaction is: 
(A) 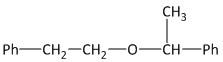
(B)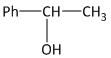
(C) Ph−CH2−O−CH2−CH2−Ph
(D) 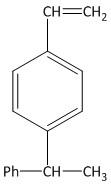
Solution
The NBS is a reagent used for benzylic bromination. The middle step is an elimination reaction. The final step is a hydration reaction of alkene.
Complete step by step answer:
The given starting material for the reaction sequence is ethyl benzene. It is an aromatic hydrocarbon which undergoes benzylic bromination upon heating with NBS. NBS is a brominating agent which contains bromine radicals. The bromine radical reacts with the hydrocarbon to produce more stabilized benzyl bromide. Thus the product of the reaction is (1-bromoethyl)benzene. The reaction is shown as
Step I: 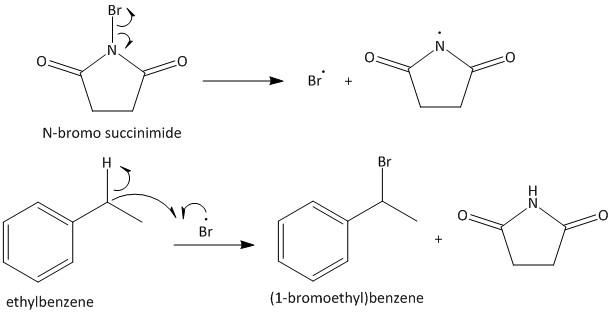
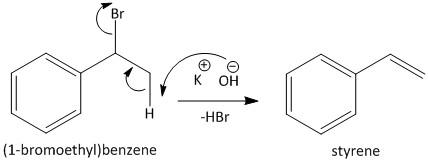
In the second step the alcoholic KOH is used to abstract the hydrogen from the methyl group of the benzyl bromide. The abstraction of the hydrogen from the compound results in dehydrobromination and produces styrene. The reaction is shown as
Step II:
The third and the final step is the hydration of styrene molecules in presence of aqueous acid. At first the acid protonated the double bond of the styrene molecule to produce a more stable 2ocarbocation (secondary). This then undergoes addition of water molecules to generate 1-phenylethan-1-ol. The reaction is shown as
Step III:
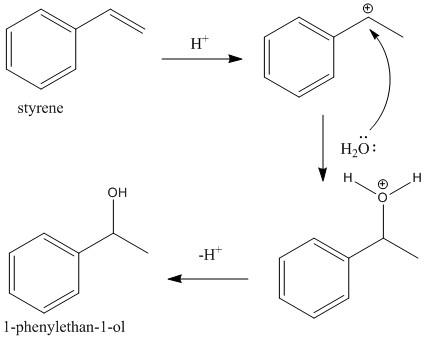
Hence the end product ‘W’ of the given sequence of reaction is 1-phenylethan-1-ol, i.e. option B is the correct answer.
So, the correct answer is Option B.
Note: The reaction conditions and the abbreviations are to be kept in mind. The alc. is used for alcoholic KOH and aq. is used for aqueous KOH. Alc. KOH will result in elimination of the product but the aqueous KOH is used for hydroxylation i.e. addition of OH.
