Question
Question: The end product of the following reaction would be: 
A.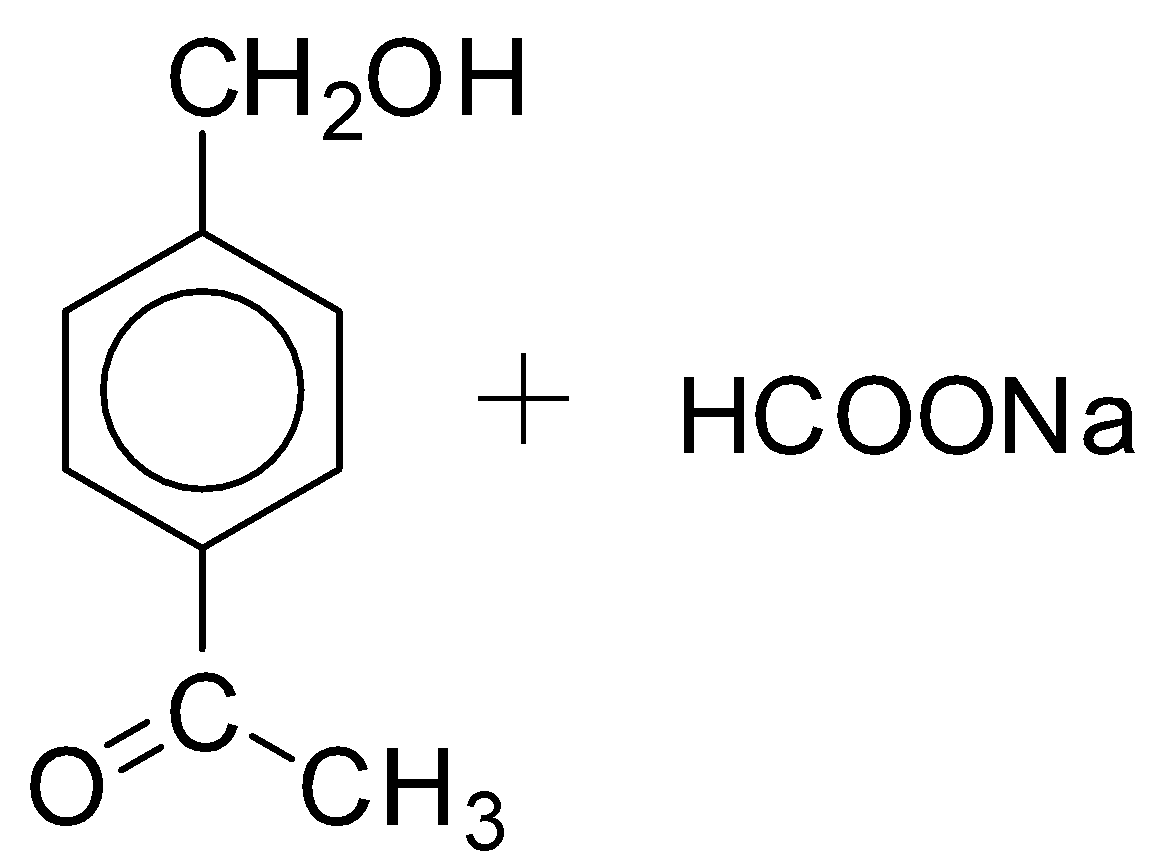
B.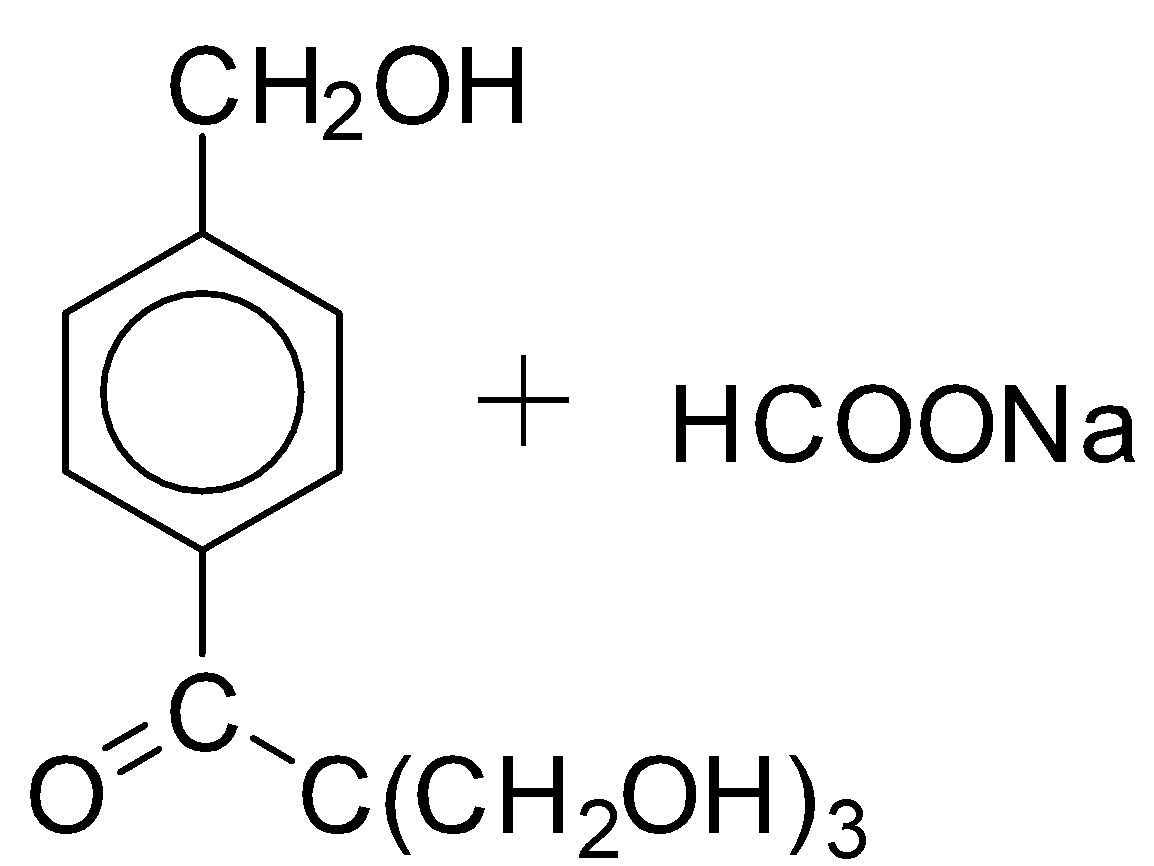
C.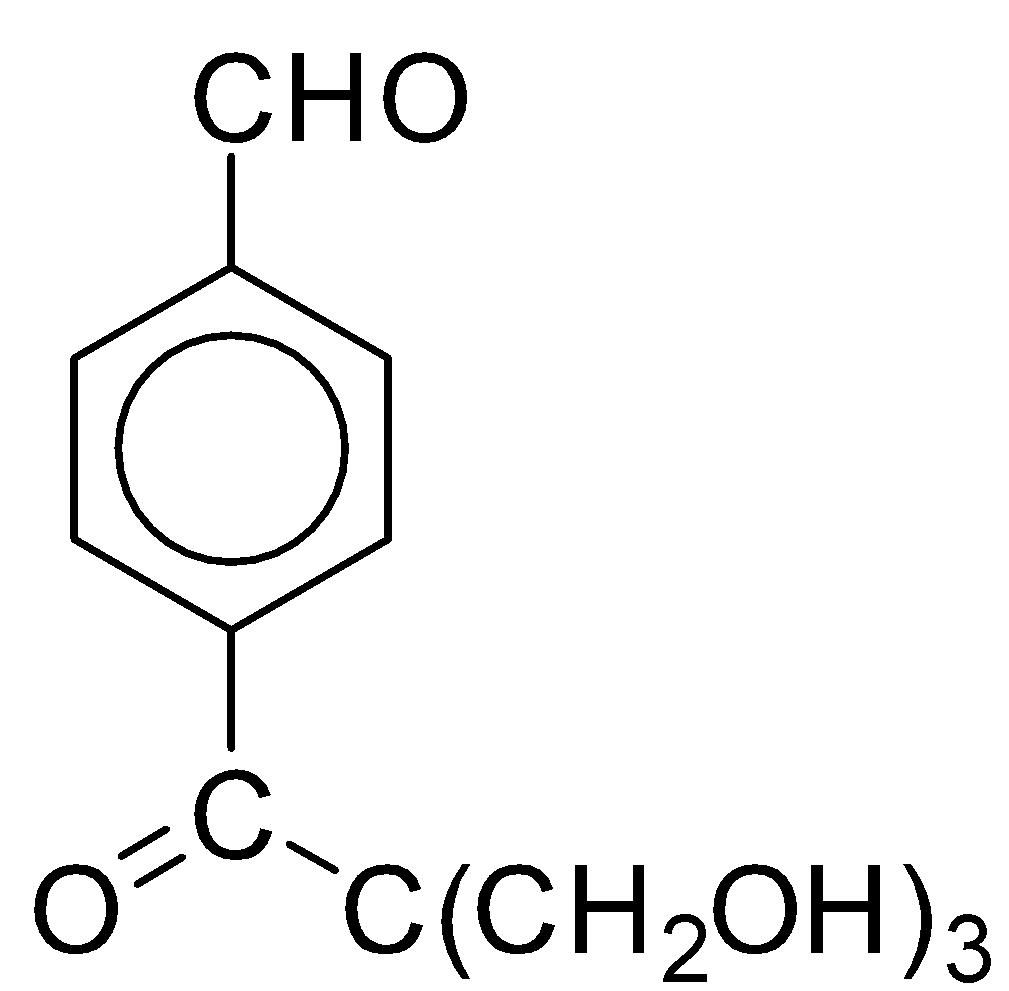
D.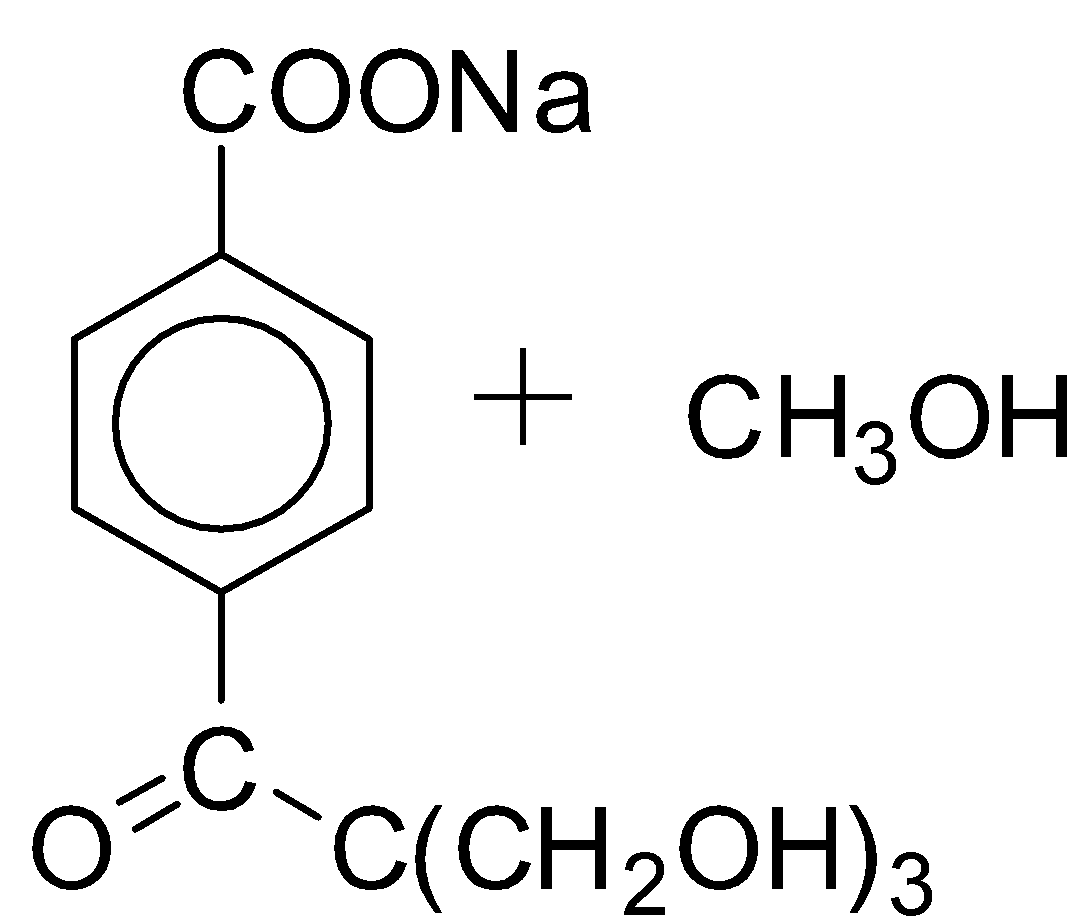
Solution
The IUPAC nomenclature of the compound given is 4 – acetyl benzaldehyde. Thus, we can say that this compound is an aldehyde. Along with the six carbons in benzene, the compound consists of nine carbons in total. In addition to that, it has eight hydrogens and two oxygen in it. This aldehyde reacts with NaOH more than HCHO. The IUPAC name of acetaldehyde is ethanal. Hence, we can say that the molecular formula of ethanal is CH3CHO. Thus, we can say that the combination of acetaldehyde and benzene is the compound acetyl benzaldehyde.
Complete answer:
-When 4 - acetyl benzaldehyde reacts with the compound of sodium hydroxide (i.e., NaOH) in the presence of formaldehyde (HCHO) in excess, it leads to the formation of sodium Acetate.
-Thus, we can say that the reaction takes place as below:
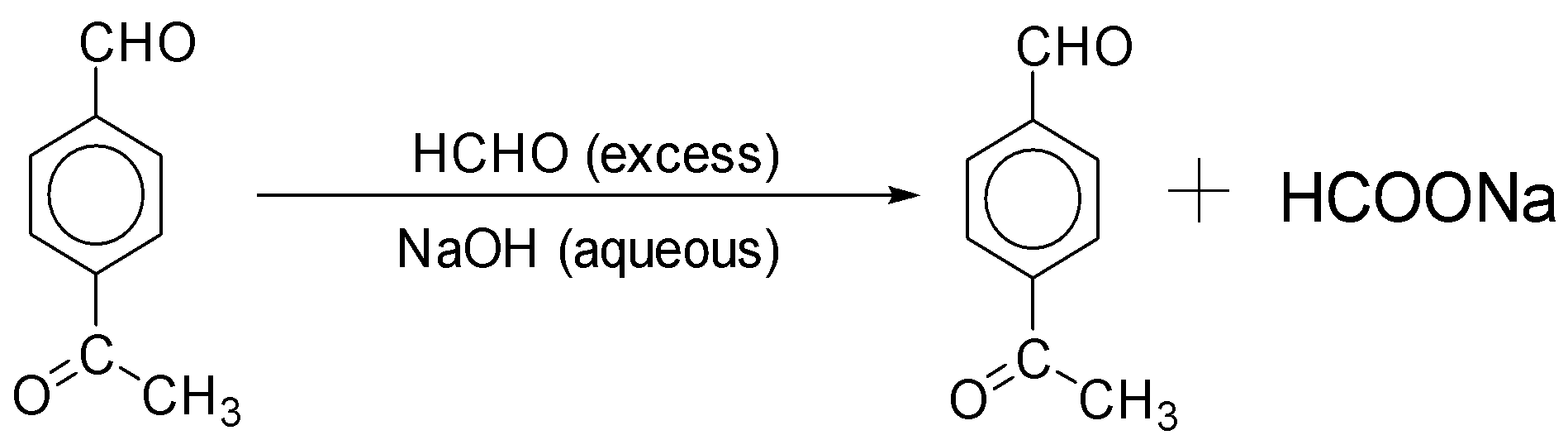
-Thus, we can assume that this reaction's products will be 4 - acetyl benzaldehyde along with sodium Acetate.
-One can say that the reaction of 4 - acetyl benzaldehyde with sodium hydroxide (i.e., NaOH) in the presence of formaldehyde (HCHO) in excess is an example of the aldol condensation reaction. Such reactions are given by aldehydes (- CHO) or ketones ( R=O), which have an alpha hydrogen atom.
Thus, the correct option is (A).
Note:
As we already know that placing the molecules of acetaldehyde in different positions in a benzene molecule gives acetyl benzaldehyde. In general, the acetaldehyde molecules are placed in Thus, and we can say that the molecular formula of this compound is C9H8O2. Generally, the acetaldehyde is placed in the third and fourth position of benzene, leading to the formation of 3 - acetyl benzaldehyde and 4 - acetyl benzaldehyde, respectively.
