Question
Question: The end product D what is the reaction? 
Solution
This question gives the knowledge about the dehydration reaction, addition reaction and Grignard’s reagent reaction. Dehydration reaction is the reaction in which release of water molecules takes place with the help of a dehydrating agent.
Complete step-by-step answer: It is a multistep reaction. A multistep reaction is the reaction which completes through various steps. In this reaction, at each step various name reactions are taking place.
The detailed mechanism of the reaction is as follows:
In the first step a dehydration reaction is taking place. Dehydration reaction is the reaction in which release of water molecules takes place with the help of a dehydrating agent. In this step, tertiary butanol reacts with aluminum oxide in the presence of heat to form alkene.
In the second step, the resultant alkene undergoes addition reaction with hydrogen bromide in the presence of peroxide using anti-markovnikov rule. In this step the bromide group attaches to the less hindered site.
In the third step, elimination reaction takes place. In this step the bromide group gets eliminated by the action of aqueous sodium hydroxide.
In the fourth step, Grignard’s reaction takes place. In this step the negative charge present on the benzene ring forms a bond with the positive charge on the carbon atom of another molecule.
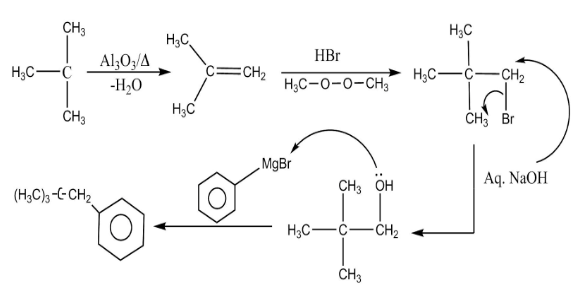
The end product D of the reaction is
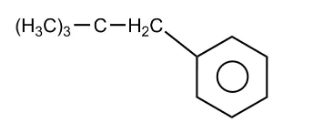
Hence, option 2 is the correct option.
Note: Dehydrating reaction results in the removal of water molecules when two molecules react with each other. Elimination reactions generally leads to the formation of alkene by the removal of good leaving groups such as bromide, chloride and so forth.
