Question
Question: The empirical formula of natural rubber is: A. \({\left( {{C_3}{H_5}} \right)_n}\) B. \({\left( ...
The empirical formula of natural rubber is:
A. (C3H5)n
B. (C5H8)n
C. (C5H3)n
D. (C8H5)n
Solution
We can say the empirical formula of a compound is the simplest whole number ratio of each type of atom in a compound. It can be the same as the compound’s molecular formula but not always. We can calculate an empirical formula from information about the mass of each element in a compound or from the percentage composition.
Complete step by step answer:
In our daily life, we come across many rubber products. Some common rubber-based objects that we use in our day-to-day lives include rubber gloves, band, and rubber footwear.
We have to know that rubber items contain the ability to regain their shapes after being stretched or distorted, and this is the reason why we call rubber as an elastomer.
Rubber is an elastic substance which can be obtained both naturally (natural rubber) or artificially in laboratories (synthetic rubber-like butyl rubber, neoprene, etc.)
Natural rubber and synthetic rubber are two primary types of rubber.
We can say natural rubbers are the elastomers that are obtained naturally. Natural rubber is composed of solid particles suspended in a milky white liquid known as latex that drips from the bark of certain tropical and subtropical trees.
We can say synthetic rubbers are obtained from petroleum and natural gas. It is derived by polymerization of 1, 3 – butadiene derivatives or by copolymerization of 1, 3 – butadiene along with unsaturated monomers. Some of the synthetic rubbers are Buna-S, Buna-N, and Neoprene etc.
We have to know that natural rubbers are formed by the polymerization of isoprene. Isoprene is a monomer. We can draw the structure of isoprene as,
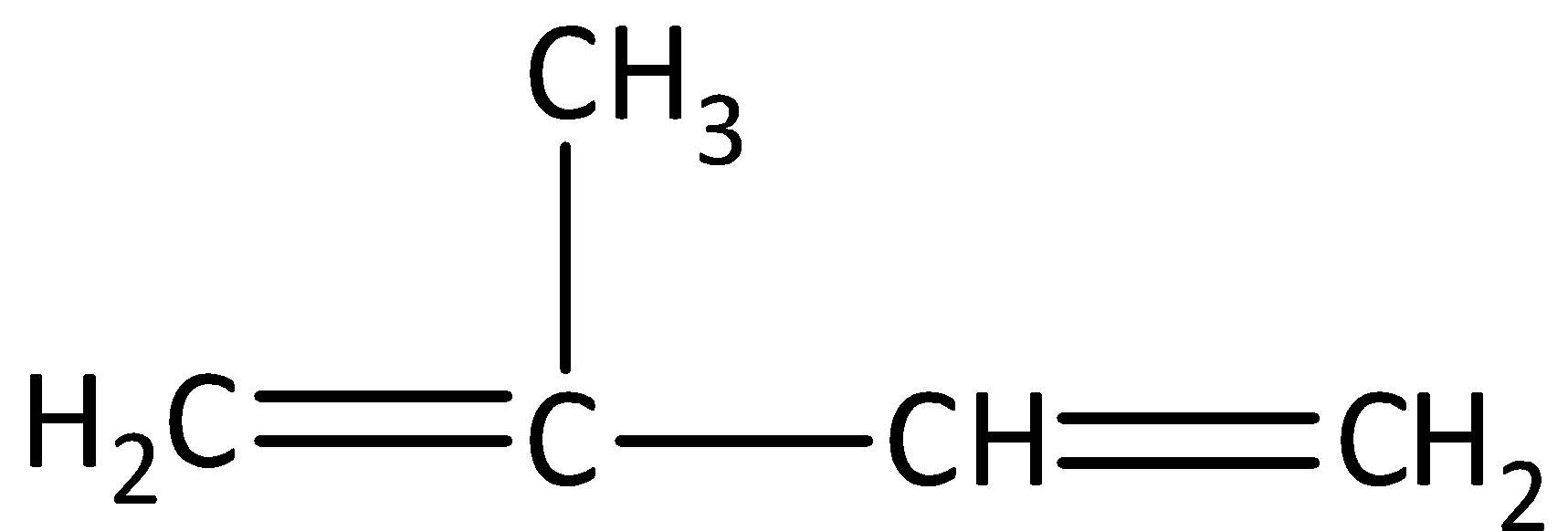
We can see that in isoprene, the number of carbon atoms is five and the number of hydrogen atoms is eight. Therefore, the empirical formula of isoprene is (C5H8)n
Option (B) is correct.
Note:
We can use rubber for lining chutes, bins and industrial mixers. In the clothing industry, rubber is used as wetsuits and in expandable clothes like gym and cycling shorts etc. Rubbers are used for flooring purposes. In the automobile industry, rubber use can be witnessed in tires, padding in brakes, airbags, seats, and roof etc. We can also call isoprene as 2-methyl-1,3-butadiene. It is a volatile liquid and appears colourless.
