Question
Question: The empirical formula of natural rubber is: (a)- \({{({{C}_{3}}{{H}_{5}})}_{n}}\) (b)- \({{({{C...
The empirical formula of natural rubber is:
(a)- (C3H5)n
(b)- (C5H8)n
(c)- (C5H3)n
(d)- (C8H5)n
Solution
The empirical formula of a chemical compound is calculated with the help of two factors i.e., the percentage composition of elements and atomic mass of the element. The empirical formula of a compound can be calculated with the molecular formula by converting it into the simplest whole-number ratio.
Complete step by step answer:
Let us first study the empirical formula and steps to calculate it:
The empirical formula is the chemical formula that tells the simplest whole-number ratio of the atoms of all elements present in one molecule.
It is deduced by- (a) percentage composition of elements (b)- atomic masses.
There are some steps for calculating the empirical formula:
i)- To convert the mass percent into grams. The given mass percentage represents the masses of the element in grams.
ii)- To calculate the number of moles. We have to divide each element’s percentage by its atomic mass.
moles of the element= At. mass of the elementmass of the element
iii)-To calculate the simplest molar ration. Divide the moles obtained in step (i) by the smallest quotient or the least value from amongst the values obtained for each element. This gives the simplest molar ratio.
iv)- To calculate the simplest whole number ratio. The simplest molar ratios, as calculated in step 2, are generally whole numbers. If not, then (a) raise the values to the nearest whole number, or (b) multiply the simplest atomic ratio by a suitable integer.
v)- To write the empirical formula. Insert the numerical value of the simplest whole number.
Natural rubber is a compound that has a very high number of molecules and it is a polymer. It is made from isoprene monomer, whose molecular formula is C5H8and the structure is given below:
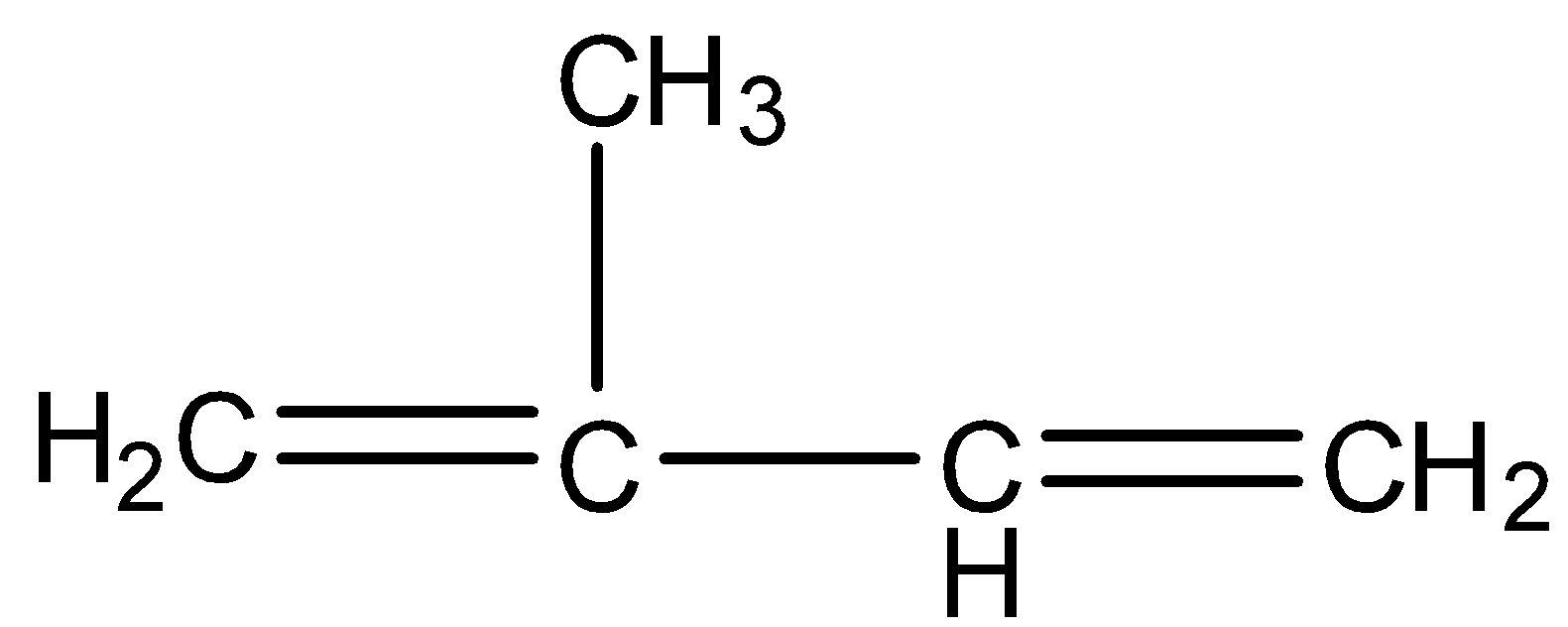
So, the repeating unit of natural rubber is C5H8, therefore the empirical formula of natural rubber will be (C5H8)n.
Hence, the correct answer is an option (b)- (C5H8)n
Note: The simplest molecular ratio should always be converted into the whole number. If not converted this would give the exact or true formula which gives the molecular formula. Don't get confused between molecular and empirical formulas.
