Question
Question: The empirical formula of a monobasic acid is \(C{{H}_{2}}O\). The vapour density of its ethyl ester ...
The empirical formula of a monobasic acid is CH2O. The vapour density of its ethyl ester is 44. What is the molecular formula of the acid?
A. C2H4O2
B. CH2O2
C. C2H2O2
D. C3H6O3
Solution
Think about the formula that relates vapour density and molecular weight. Relate and visualize the structures and of the respective ester as well as the acid. Find weight of alkyl group attached
Complete answer:
We know the general formulae and structures that define an acid and an ester. Let us consider the structures of the acid with the given empirical formula along with the structure of its ethyl ester.
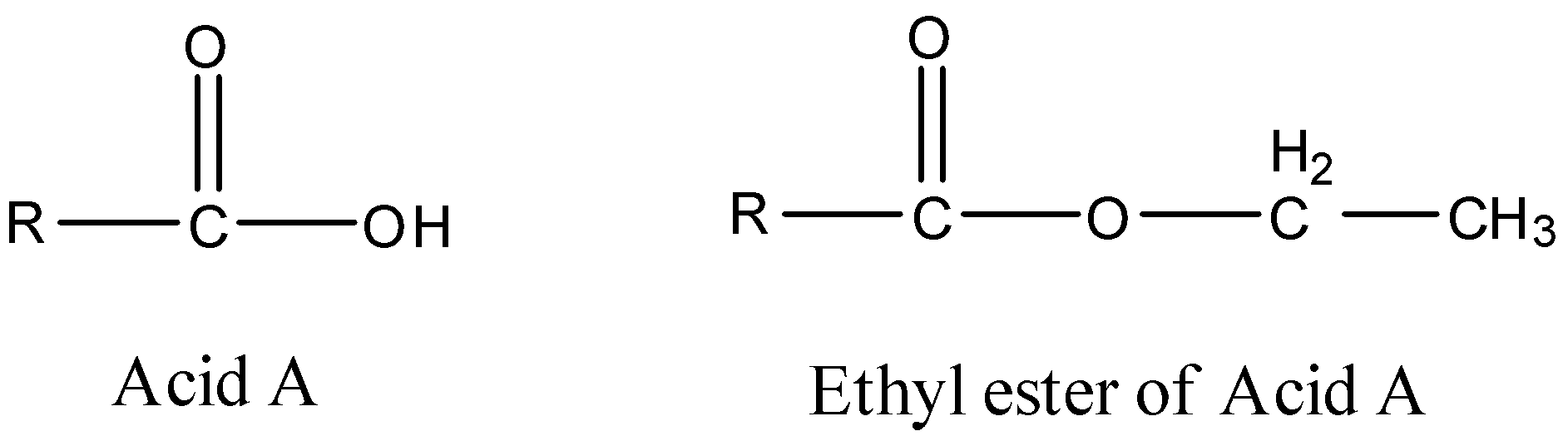
Here, we can see that the acidic hydrogen atom on the acid is replaced by the ethyl group of the aster, and the only unknown left is the parent alkyl group on both the molecules. To calculate that, we will first have to calculate the molecular mass of the ester.
We know that the molecular mass is twice the vapour density. So:
molecular mass of ethyl ester of acid A = 2×vapour density
We have been given the vapour density of the ethyl ester of acid A as 44, so its molecular weight will be:
