Question
Question: The element Z = 114 has been discovered recently. It will belong to which of the following family/gr...
The element Z = 114 has been discovered recently. It will belong to which of the following family/group and electronic configuration?
a.) Nitrogen family, [Rn]5f146d107s27p6
b.) Halogen family, [Rn]5f146d107s27p5
c.) Carbon family, [Rn]5f146d107s27p2
d.) Oxygen family, [Rn]5f146d107s27p4
Solution
Recall the elements above 100 and last element i.e. 112 or 113 to which category it belongs and then you can easily identify it and by using Aufbau’s principle you can find the electronic configuration.
Complete Solution :
- In the modern periodic table, the physical and chemical properties of the elements are the periodic functions of their atomic weights and the long period table has been divided into the periods (horizontal rows) and groups (vertical columns). In terms of the electron configuration, a group consists of a series of elements which have the same outermost electronic configurations but they have different total numbers of electrons. In the periodic table, there are a total of 18 groups.
- The elements belonging to the groups 1 and 2 belong to s-block elements and have the general electronic configuration as ns1−2 and the elements belonging to the groups 13 and 18 belong to p-block elements and have the general electronic configuration as ns2np1−6. From the statement, we can see that the outermost configuration is the same as the p- block elements and thus, an element with atomic 114 is a p- block element.
- The element uranium having the atomic number Z = 92 have been irradiated with many slow neutrons and many new radioactive elements up to Z = 112 and earlier it has been named as ununbium but now its names have been discovered and is now as Copernicium (Cn) which belongs to d- block elements category and has the outer electronic configuration as [Rn]5f146d107s1 and belongs to the family of Zinc. After the zinc family, the element no 113 belongs to the next family i.e. the Boron family, so definitely the element with Z = 114 belongs to the carbon family and 14th group of the period table and has the outer configuration as ns2np2.
- Now, we have to write the electronic configuration of this element, which we will write on the basis of Aufbau’s principle which states that the electrons are filled in the increasing order of their energies i.e. the electrons having the lower energies are filled first. The increasing orders of energies are as follows:
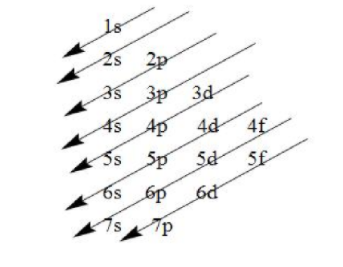
- The order in which the energies of the orbitals are increased and filled is:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f ,5d, 6p, 7s, 5f, 6d, 7p and so on.
- By this, we calculate the electronic configuration and thus it comes out to be [Rn]5f146d107s27p2.
So, thus the element = 114 belongs to 14 the group, carbon family and has the electronic configuration as[Rn]5f146d107s27p2.
Note: While writing the electronic configuration, keep in mind that the electrons are filled in the orbitals having lower energy followed by orbitals having higher energy as indicated by the arrows above.
