Question
Question: The electrostatic force of attraction between cation and anion is called___________....
The electrostatic force of attraction between cation and anion is called___________.
Solution
Hint : cation and anions are ions both having different net electrical charges. Cations are ions having a net positive charge and anions are ions having a net negative charge. As both ions have opposite charges, they attract each other and form bonds.
Complete Step By Step Answer:
When cation and anion come close to each other, having opposite net electric charges they both experience a force of attraction to form a bond, this force of attraction is called an electrostatic force of attraction. It is just like how the two opposite poles of a magnet attract each other. And the bond that the cation and anion form is an ionic bond. This force of attraction holds them together as a unit.
The ionic bond which is formed between the cation and anion is very strong. In this, there is a complete transfer of electrons from one molecule to the other. There is a huge electronegativity difference between the two ions when there is a formation of this bond.
To understand this we can take an example of a simple molecule: NaCl
So in Na there is 1 electron in the outermost shell and in Cl there are 7 electrons in the outermost shell. It will be difficult for Cl to transfer 7 electrons so Na transfers it 1 electron to Cl and there is a bond formed between them.
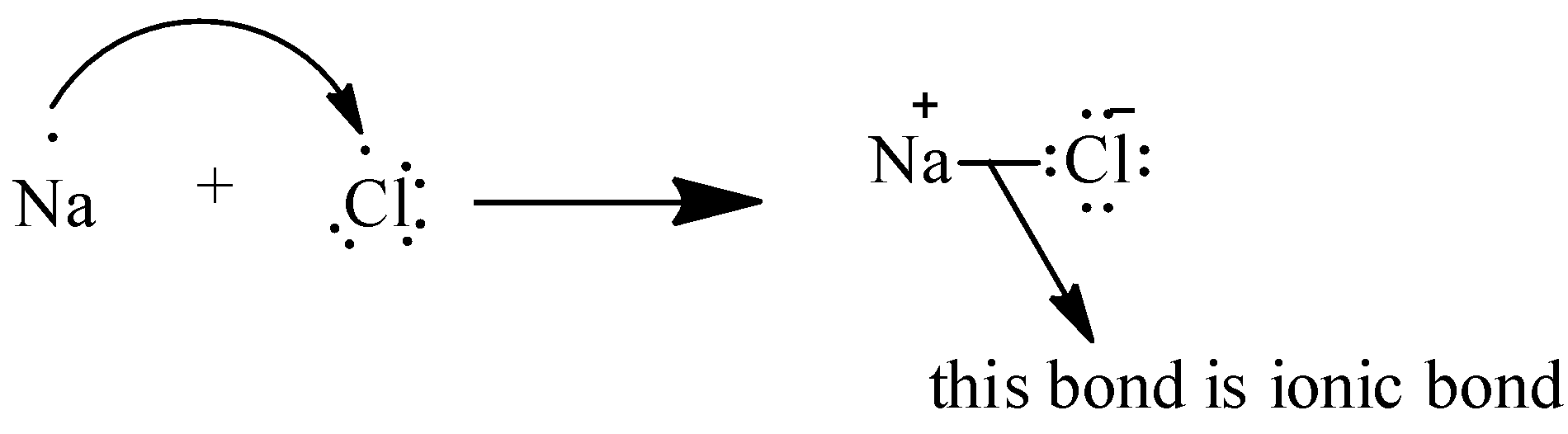
As we can see in the above picture, bond formation between Na and Cl which results in the formation of NaCl molecule. And the force of attraction with which the bond is formed is called the electrostatic force of attraction.
Note :
In physics, this electrostatic force of attraction is also called coulomb’s law, named after French physicist Charles-Augustin de Coulomb. And this force of attraction between two oppositely charged bodies is calculated using formula F=k\difracq1q2r2 .
