Question
Question: The electronic structure of the \(S{{O}_{2}}\) molecule is best represented as a resonance hybrid of...
The electronic structure of the SO2 molecule is best represented as a resonance hybrid of
Equivalent structures.
A) 4
B) 2
C) This molecule does not exhibit resonance
D) 3
Solution
In SO2 molecule there are two types of pi-bonds.
Total number of valence electrons we have are 18 electrons.6 electrons from S atom and 6 electrons from each O atom.
- S atoms possess a positive charge in their equivalent structure.
Complete Solution :
So the question is about that number of resonance structure that supports the actual structure of SO2 molecule and helps to explain every property of the molecule.
- To draw resonance structure of SO2, we have 18 e− and there is positive charge in S atom and negative charge in one of the O atom in the resonance structure.
- Actually there are three Lewis structures for SO2 molecules, but only two of them satisfy the experimental data and the third one only supports the theoretical part.
Now let’s draw the resonance structure of SO2
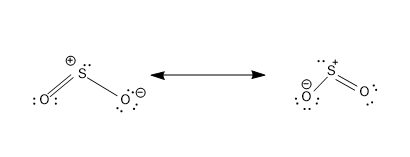
- These structures have a count of 18e−, i.e 6 e− from three bonds and 12e− are distributed as lone pairs in three atoms.
- The structures consist of proper formal charges –Negative formal charge on the most electronegative O atom and positive formal charge on the comparatively less electronegative S atom.
- These two structures are equivalent and will equally contribute to the hybrid structure of SO2
In that case the hybrid structure must be
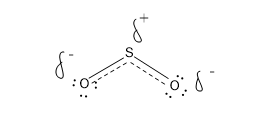
Here there is a partial negative charge on the O and a partial positive charge on the S.
The negative charge is split between two O.
So, the correct answer is “Option B”.
Note: The third Lewis structure of SO2 is,
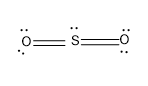
This structure is stable for the theory aspect, but does not produce evidence for experimental data.
