Question
Question: The electronic configuration which obeys Hund’s rule for the ground state of a carbon atom? A.
B.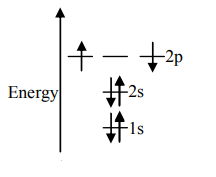
C.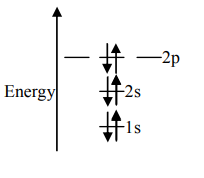
D.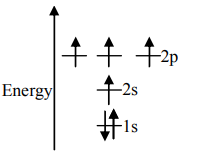
Solution
Hint : As per Hund’s rule all every orbital is singly filled before double occupancy takes place. All electrons in the single occupancy have the same spin; this gives maximum total spin. In half-filled orbits, electrons will not pair with any other electron as it has to fill all the orbits singly first.
Complete Step By Step Answer:
Unpaired electrons present in the orbit are at the ground state. As per Hund’s rule of maximum multiplicity, the term with maximum multiplicity falls lowest in the energy. Now as per the rule all the orbits should be first singly filled in option A the configuration is 1s22s22p2, here as we can see the 1sorbit is completely filled with opposite charges, also the 2s orbit is completely filled with opposite charge but the 2p orbital is filled with an only single charge as it has to singly fill all the orbits first. In 2p the orbit is filled with the same charge that is either −or +. This completely follows Hund’s rule. While in option B first and the last orbit is singly filled keeping the center orbit empty violating Hund’s rule of maximum multiplicity. Similarly in option C the center orbit is completely filled keeping the other orbits empty.
Therefore the correct option is A.
Note :
Hund’s rule is mainly used in chemistry, quantum physics, and spectroscopy. As per Hund’s rule of maximum multiplicity pairing in p d, f orbitals cannot occur until all the orbits are first singly filled. Electrons repel each other as they are negatively charged.
