Question
Question: The electronic configuration of nitrogen is 2, 5. How many electrons in the outer shell of the nitro...
The electronic configuration of nitrogen is 2, 5. How many electrons in the outer shell of the nitrogen atom are not involved in the formation of a nitrogen molecule?
Solution
While the formation of the nitrogen molecule, each nitrogen atom is deficient of 3 electrons to complete their octet. So, each atom will share 3 electrons to form the bond and the rest of the electrons will not be involved in bond formation.
Complete step by step solution: The nitrogen belongs to group 15 of the periodic table and its electronic configuration is –
N→1s22s22p3 or [He]2s22p3
We can see that the inner 1s – orbital is fully filled, so it will not take place in bonding. The outermost orbital of nitrogen contains a total of 5 electrons (2s22p3) . The nitrogen needs 3 more electrons to complete its octet.
Now, when two atoms of nitrogen approach each other, they both equally share three electrons each and form a triple covalent bond to complete their octet as shown below.

The electronic configuration of the system will look like this:
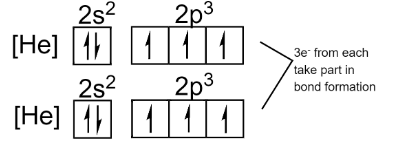
Thus, we can see that out of 5 valence electrons only 3 are involved in the bonding during the formation of a nitrogen molecule. So, the remaining 2 electrons remain intact in each nitrogen atom.
Hence, two electrons in the outer shell of each nitrogen atom are not involved in the formation of a nitrogen molecule.
Note: The nitrogen forms a covalent bond with another nitrogen atom by sharing its 3 valence electrons. It cannot completely accept or donate electrons to form an ionic bond due to the presence of stable half-filled p-orbital in its valence shell.
