Question
Question: The electronic configuration of helium is \(1{s^2}\) and of oxygen is: (A) \(1{s^2},2{s^2},2{p^5}\...
The electronic configuration of helium is 1s2 and of oxygen is:
(A) 1s2,2s2,2p5
(B) 1s2,2s2,2p4
(C) 1s2,2s2,2p3
(D) 1s2,2s2,2p6
Solution
The electronic configuration of any element is written in the order of increasing energies of the principal shells . Before writing electronic configuration of any element we should know its atomic number which is equal to the number of electrons present in the atom .
Complete step by step answer:
Oxygen is the eighth element with a total of electrons. In writing the electronic configuration for oxygen the first two electrons will go in the 1s orbital.
Since, 1s can only hold two electrons, the next 2 electrons for ‘ 0 ’ go in the 2s orbital.
The remaining four electrons will go in the 2p orbital.
Therefore the ‘ 0 ’electronic configuration will be 1s2,2s2,2p4
Electronic configuration chart
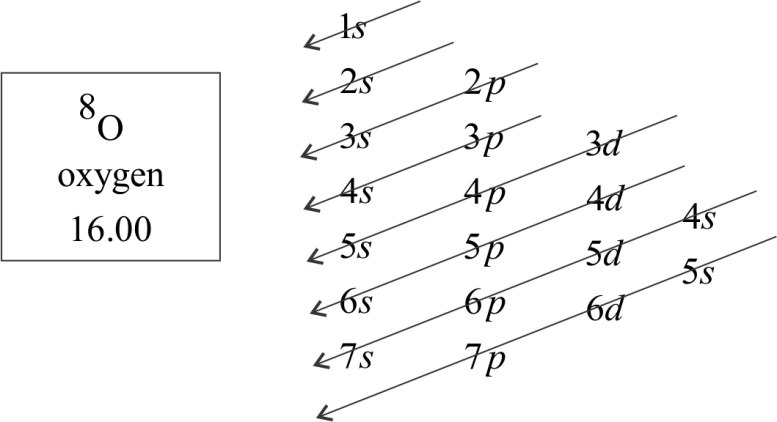
The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an atom. This makes it easier to understand and predict how atoms will interact to form chemical bonds.
Additional information:
Aufbau principle: It gives a sequence in which various subshells are filled up depending on the relative order of the energies of various subshells. The subshell with minimum energy filled up first when this subshell obtained maximum quota of electrons then the next subshell of higher energy starts filling.
Note:
The orbitals available in the subshell are first filled singly with parallel spin electron before they begin to pair this means that pairing of electron occurs with the introduction of second electron in ‘s’ subshell, fourth electron in ‘ p ’ subshell, sixth electron in ‘ d ’ subshell and eighth e−in ‘f ’ subshell.
