Question
Question: The electron dot structure of \(B{{F}_{3}}\) is: (A)  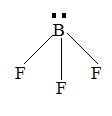
(B) 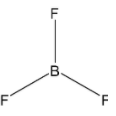
(C) 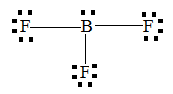
(D) 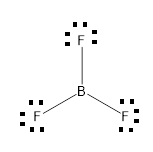
Solution
The electron dot structure is nothing but the Lewis dot structure. To answer this question we should be aware of the concept of Lewis dot structure. This concept was the basis of VSEPR theory too.
Complete answer:
Lewis dot structure is a representation of the arrangement of electrons around each atom of a compound or a molecule. This is why it is also known as electron dot structure.
Firstly, let's write the number of valence electron in boron and fluorine:
Boron = 5 = 1s22s22p1
Fluoride = 9= 1s22s22p5
The number of valence electrons in B + the number of valence electron in F×3
3 + 7 × 3
3 + 21
24
The boron is less electronegative than fluorine. So, the boron is the central atom.
Now let's draw the BF3
The boron has three valence electrons and the fluorine will have seven valence electrons around it.
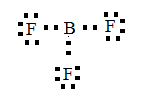
Then, join the electron and make bonds.
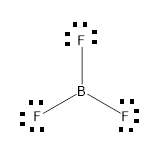
From the above drawn diagram, we can say that either option B or option D is the correct answer. The option B is just the structure of BF3 but not the dot structure of BF3. According to electron dot structure the arrangement of electrons around each atom of a compound or a molecule should be shown.
Thus, the Option D is the correct answer.
Note:
The number of valence shells in boron is so there no chance of having lone pairs on boron therefore, option A and C is the wrong answer. Option B is not the dot structure, it is the structure of BF3. Lewis dot structure too has many limitations but certain limitations are overcome by VSEPR theory.
