Question
Question: The electron dot structure for \(B{{F}_{3}}\) is: (A)- - 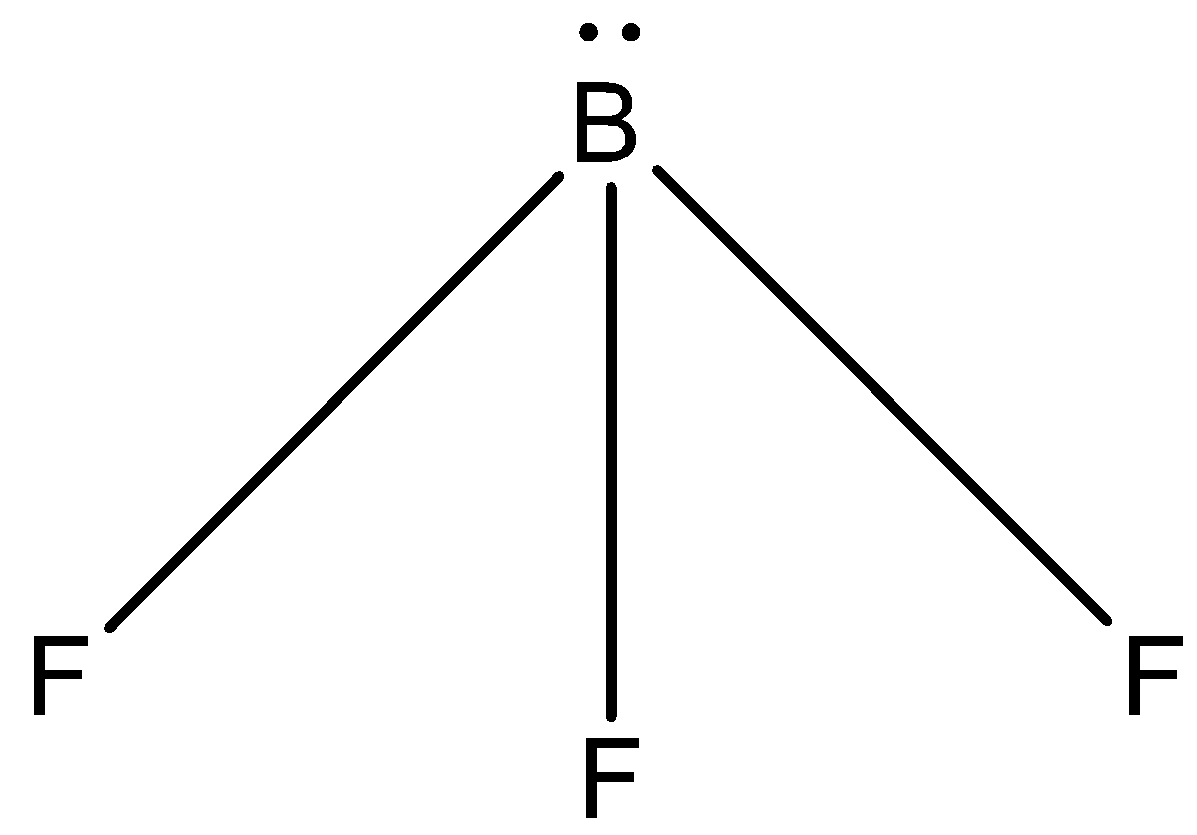
(B)- 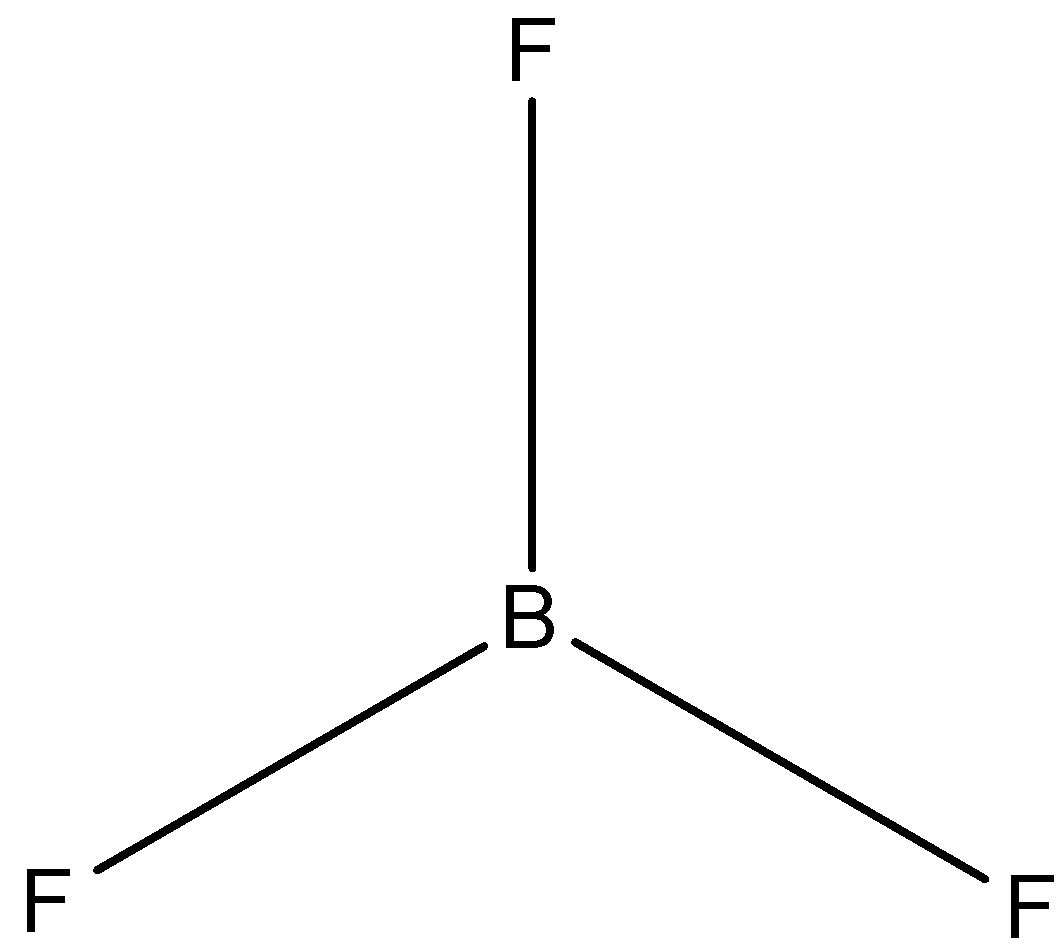
(C)- 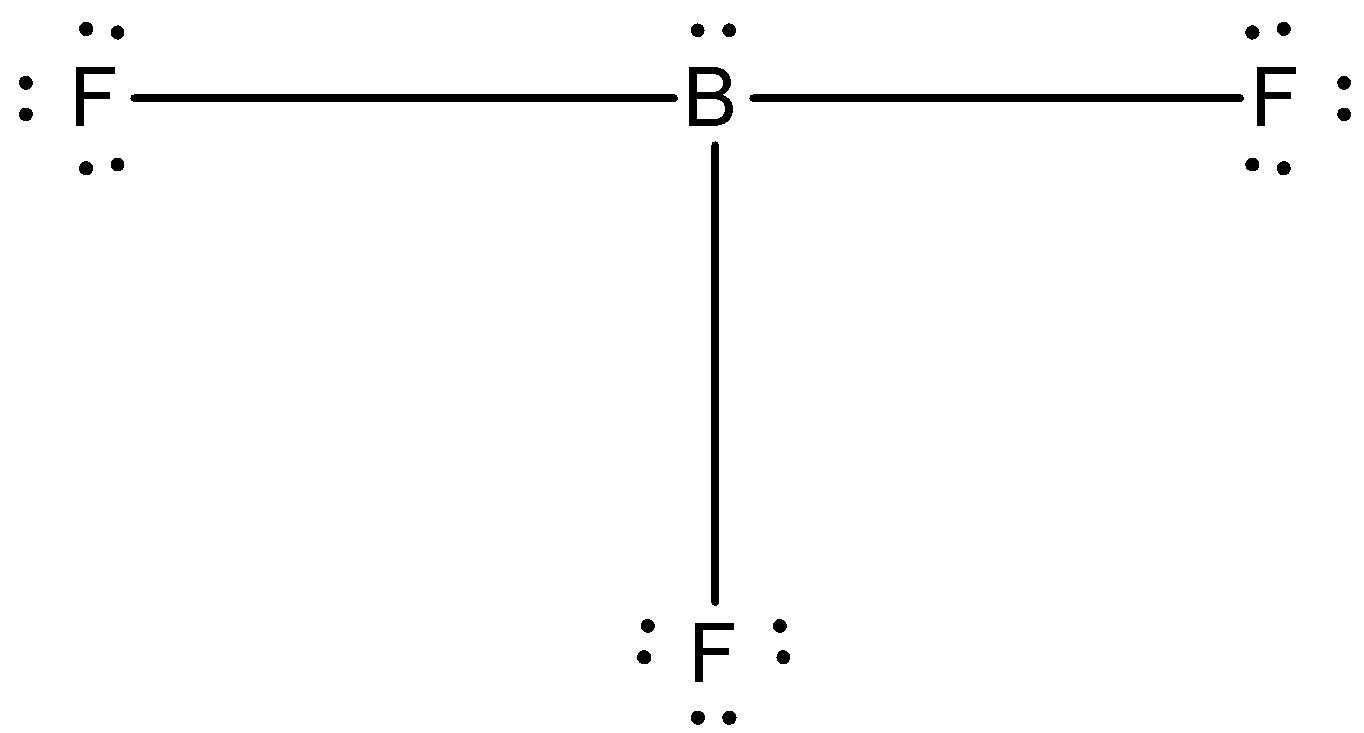
(D)- 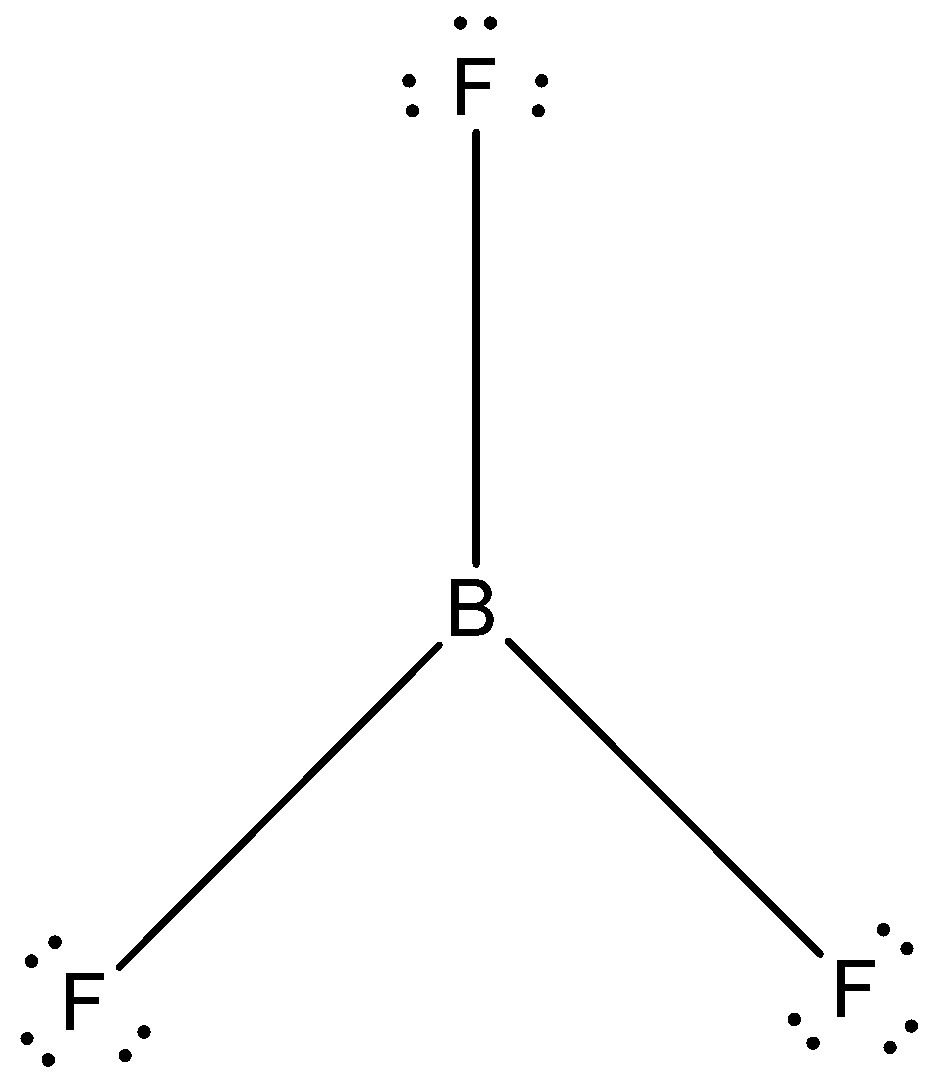
Solution
Write the electronic configuration of boron atom in its ground state. Now three electrons will be used for formation of chemical bonds with incoming fluorine atoms. Now find out the hybridization of boron atoms considering the bonds and lone pairs if present. This will help to find the structure of the compound.
Complete step by step answer:
- Boron trifluoride is an organic compound with the formula BF3. It is a pungent colourless toxic gas which forms white fumes in moist air. It is very good Lewis acid as it has a vacant orbital that can accommodate a lone pair of electrons.
- We will write the electronic configuration of boron atoms.
E.C = 1s22s22p1
Boron has 3 electrons in its valence shell. These electrons are used up in bond formation with the incoming fluorine ions. Boron has no lone pair of electrons.
- Let us determine its hybridisation.
Number of bond pairs = 3
Number of lone pairs = 0
The hybridisation becomes sp2 .
BF3 is a planar compound with hybridisation sp2 and has a trigonal planar shape.
- The Lewis dot structure of boron trifluoride is given below:
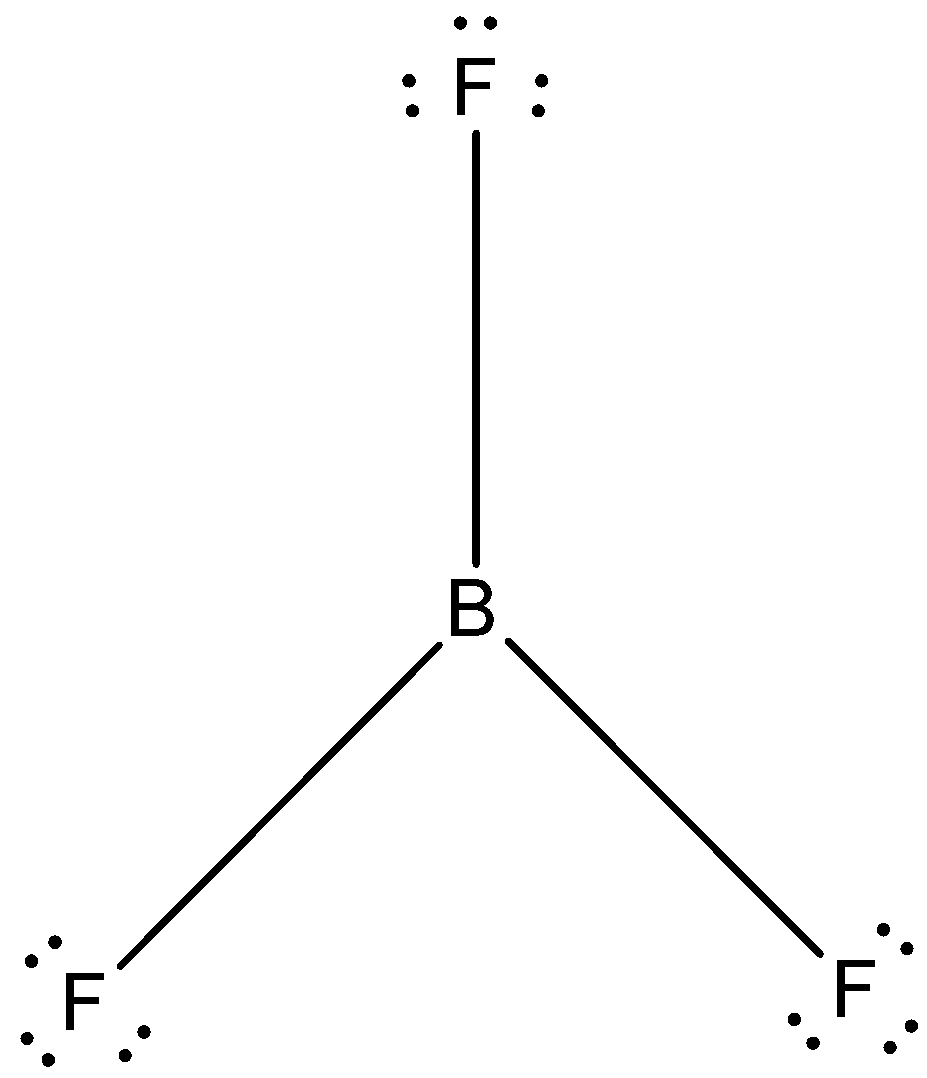
The correct answer is option “D” .
Note: Fluorine has 3 lone pairs of electrons in its valence shell. We know that boron has a vacant orbital in boron trifluoride. Due to this there is formation of a synergic bond between one fluorine atom and central boron atom. This is called back bonding and is very prevalent in boron trifluoride.
