Question
Question: The distribution of molecules with velocity is represented by the curve. Velocity corresponding to p...
The distribution of molecules with velocity is represented by the curve. Velocity corresponding to point A is:
A. M3RT
B. M2RT
C. πM8RT
D. MRT
Solution
The Maxwell-Boltzmann Distribution describes the distribution of molecular speeds. A gas, according to the Kinetic Molecular Theory of Gases, has a vast number of particles moving at high speeds. Each particle has its own speed, and each interaction between particles alters the particle speeds.
Complete step by step answer:
Individual molecules in a gas move with random speeds in magnitude and direction, while a gas with numerous molecules has a predictable distribution of molecular speeds. The Maxwell-Boltzmann distribution, named after its creators who derived it using kinetic theory, is a predictable distribution of molecular speed.
Now, coming to the question
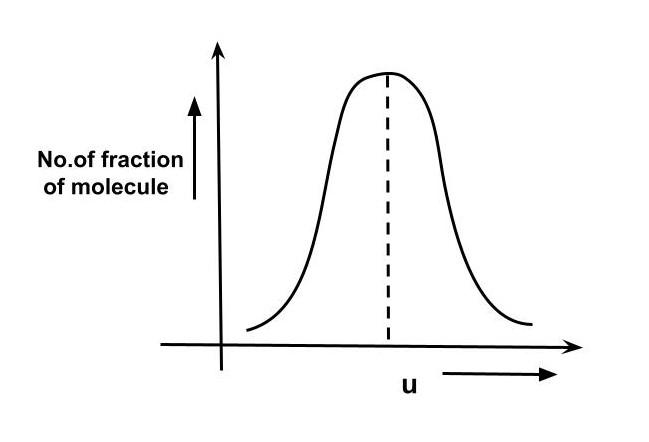
We can see in the graph that point A represents the most probable distribution of molecules hence the most probable velocity is M2RT
So the correct option is B. M2RT .
Additional Information:
The temperature and mass of the particles affect the velocity distributions. The particles get more kinetic energy as the temperature rises. When we plot this, we can observe that as the temperature rises, the Boltzmann plot expands and the relative maximum shifts to the right.
Note:
Remember that, according to the Maxwell-Boltzmann distribution law of molecular velocities of gases, all gas molecules, regardless of mass, have the same average translational kinetic energy of 23KT per molecule at any given temperature.
