Question
Question: The dissociation of nitrogen pentoxide is a first-order reaction. In the first 24 min, 75% of nitrog...
The dissociation of nitrogen pentoxide is a first-order reaction. In the first 24 min, 75% of nitrogen pentoxide is dissociated. What amount of nitrogen pentoxide will be left behind after one hour of the start of the reaction?
(A) Approximately 1 %
(B) Approximately 2 %
(C) Approximately 3 %
(D) None
Solution
A reaction that proceeds at a rate that depends linearly on only one reactant concentration is known as a first-order reaction. Other reactants can also be present in the reaction, but each will have zero order. The rate law and half-life for the first-order reaction are given below-
−dtd[A]=k[A]; t1/2=kln(2)
Complete Solution:
-Calculating the half-life period of nitrogen pentoxide-
∴t1/2=224min=12min
n = number of half-life in 1 hour (60 min) =1260=5
-Amount of substance left after 5 half-lives-
(21)5=321
Therefore, the % of the amount left =32100=3.125
So, the correct answer is “Option C”.
Note: Let us see the derivation of half-life for first-order reactions-
The time taken for the concentration of a given reactant to reach 50% of its initial concentration is known as the half-life of a chemical reaction.
- For the first-order reaction, the rate constant can be mathematically given as –
k=t2.303log[R][R0]
- From the definition of half-life,
At t=t1/2 and [R]=2[R0]
Substituting the above values in the equation for the first-order rate constant, we will get
k=t1/22.303log[R0]/2[R0]
- Rearranging the above equation to get the value of t1/2
t1/2=k2.303log(2)
As we know that the value of log(2) is 0.693.
Now, substituting the value of log 2 in the above equation, we will get,
t1/2=k0.693
-Let us now see the graphs of the expected shapes of the curves for plots of reactant concentration versus time and the natural logarithm of reactant concentration versus time for a first-order reaction-
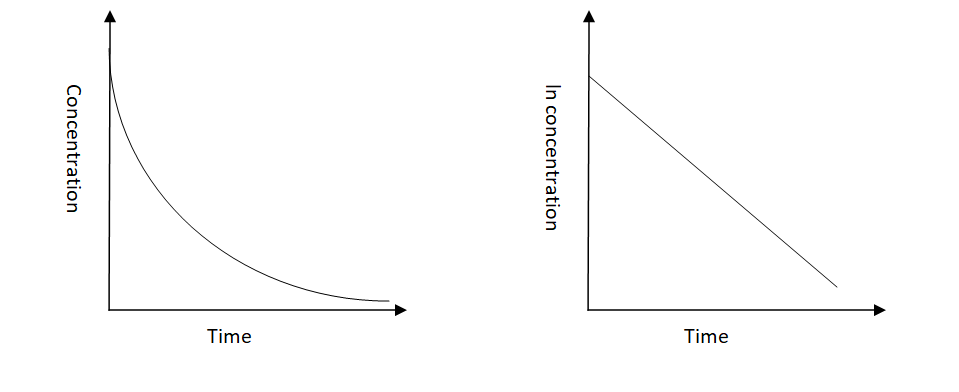
-The order for first-order reactions is always equal to 1. Following are some examples of first-order reactions-
(i) SO2Cl2→Cl2+SO2
(ii) 2N2O5→O2+4NO2
(iii) 2H2O2→2H2O+O2
