Question
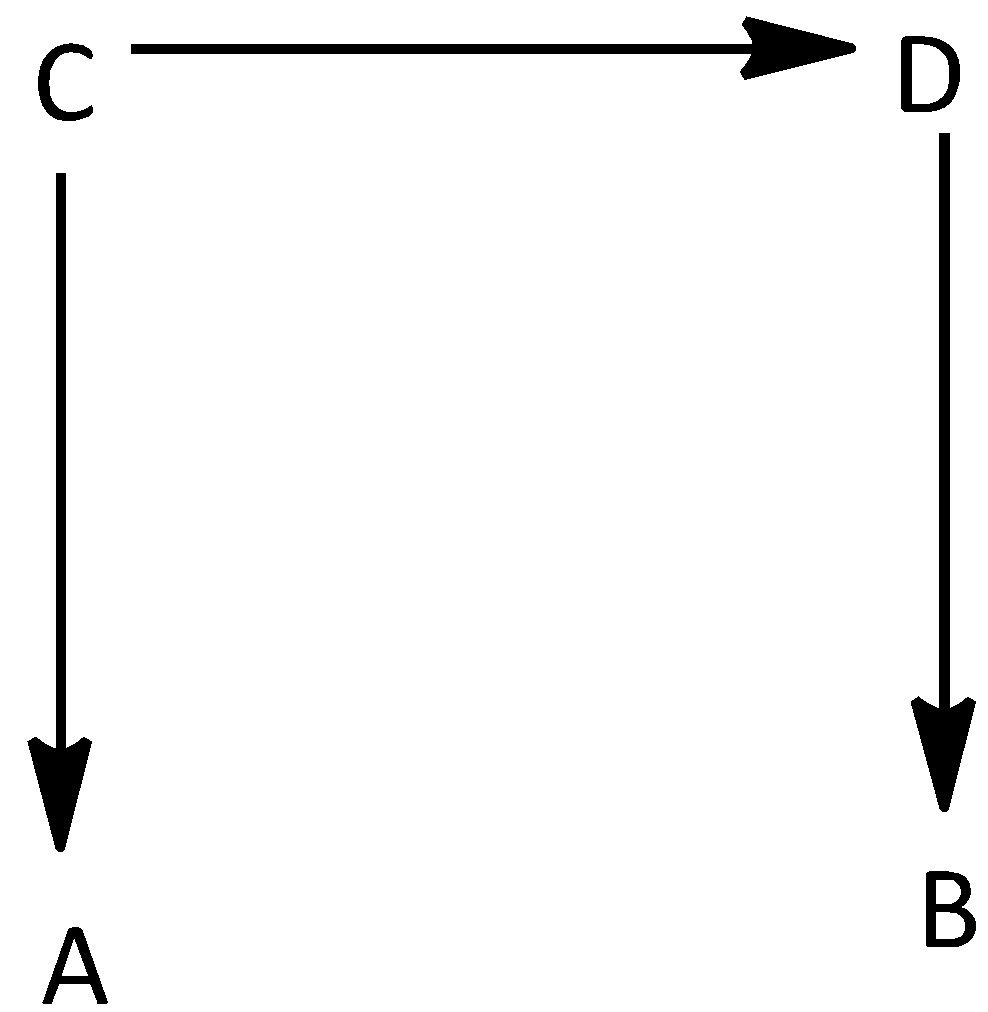
Question: The direct conversion of A to B is difficult, hence it is carried out by the following shown path- ...
The direct conversion of A to B is difficult, hence it is carried out by the following shown path-
ΔS=(A→C)=50;ΔS=(C→D)=30;ΔS=(B→D)=+20
The entropy change for the process A→B
A.100
B.−60
C.−100
D.++60
Solution
We need to know that the entropy is stated as the disorder of the system or entropy is the measure of randomness of the system. According to thermodynamics, the entropy is used to explain the behavior of a system and it is explained in terms of thermodynamic properties like pressure, temperature, and heat capacity. At the same time, the statistical definition of entropy is also explained and that is, the entropy is considered as a measure of molecular disorder. The entropy is represented in the symbol, ‘S’ and the entropy is a thermodynamic state function.
Complete answer:
As we know that the enthalpy change for the conversion of A to B is not equal to 100. Hence, option (A) is incorrect.
The change in enthalpy for the conversion of A to B is not equal to −60. Hence, option (A) is incorrect.
The enthalpy change for the conversion of A to B is not equal to −100. Hence, option (A) is incorrect.
According to the question, the direct conversion of A to B is very difficult. Therefore, it is carried out by using different paths. Here, first
A is converted to C with change in entropy, 50. The C is converted to D with change in entropy 30and the B is converted to D with entropy change, +20.
ΔS=(A→C)=50
ΔS=(C→D)=30
ΔS=(B→D)=+20
Therefore, the change in enthalpy to convert D to B is equal to −20.
Hence, the enthalpy change for the conversation of A to B is,
ΔS=(A→B)=50+30−20=60
Hence, option (D) is correct.
Note:
We need to remember that the entropy is a thermodynamic state function. Because, the entropy only depends on the state of the system and it will not depend on the path of the system. The SI of entropy is J/Kmol and the CGS unit is cal/Kmol. And the entropy is the extensive property. In an isolated system, the entropy is increasing due to its greater disorder. And the entropy is also increasing with increasing the number of products in a chemical reaction by the breakage of reactants. The entropy increases with decreasing regularity.
