Question
Question: The dipole moment of \(N{F_3}\) is smaller than. A)\(N{H_3}\) B)\(C{O_2}\) C)\(B{F_3}\) D...
The dipole moment of NF3 is smaller than.
A)NH3
B)CO2
C)BF3
D)CCl4
Solution
We know that a moment arises in any system during which there's a separation of charge is called a dipole moment. They can, therefore, arise in ionic bonds also as in covalent bonds. Dipole moments arise due to the difference in electronegativity between two chemically bonded atoms.
Complete step by step answer:
Let we see the structure of given molecules,
The structure of BF3 is,
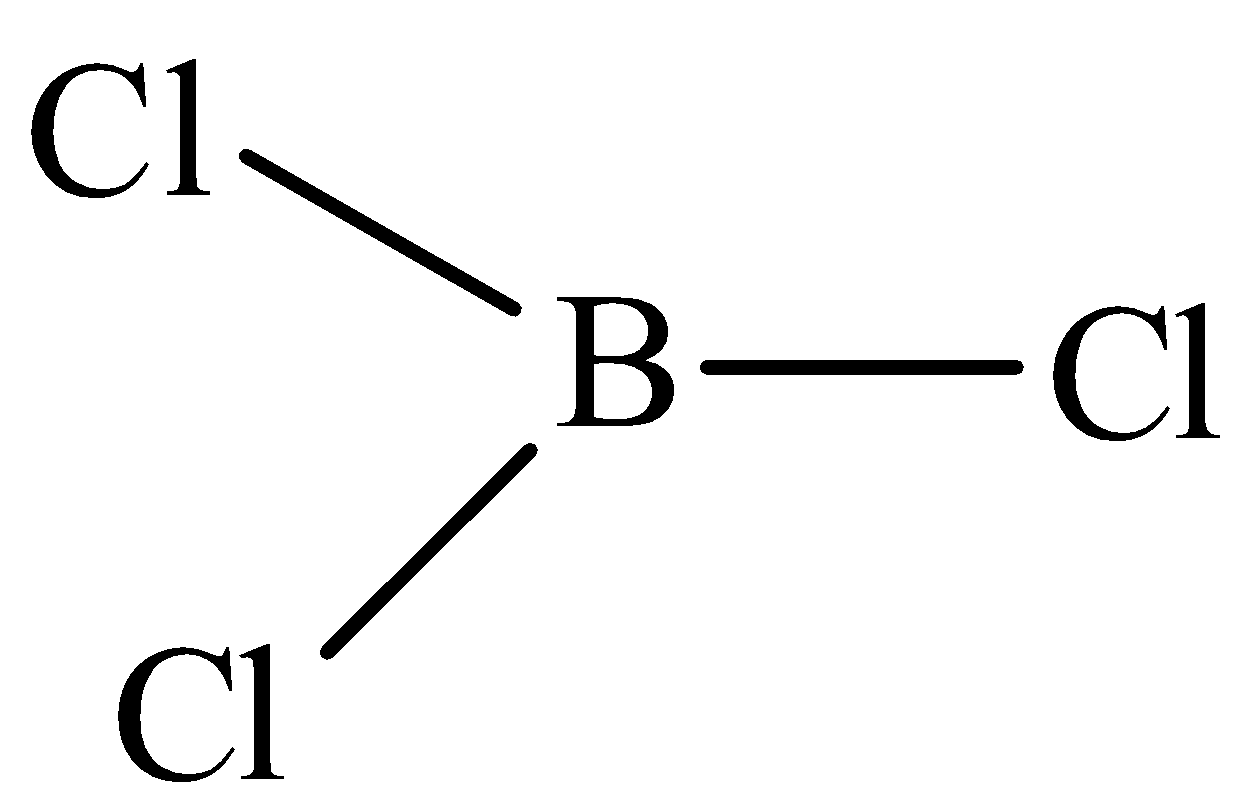
The structure of BF3 is trigonal planar with the bond angle of 120o.The dipole moment in boron trichloride is equal and in opposite direction therefore they cancel each other.
Therefore, option C is incorrect.
The structure of CCl4 is,
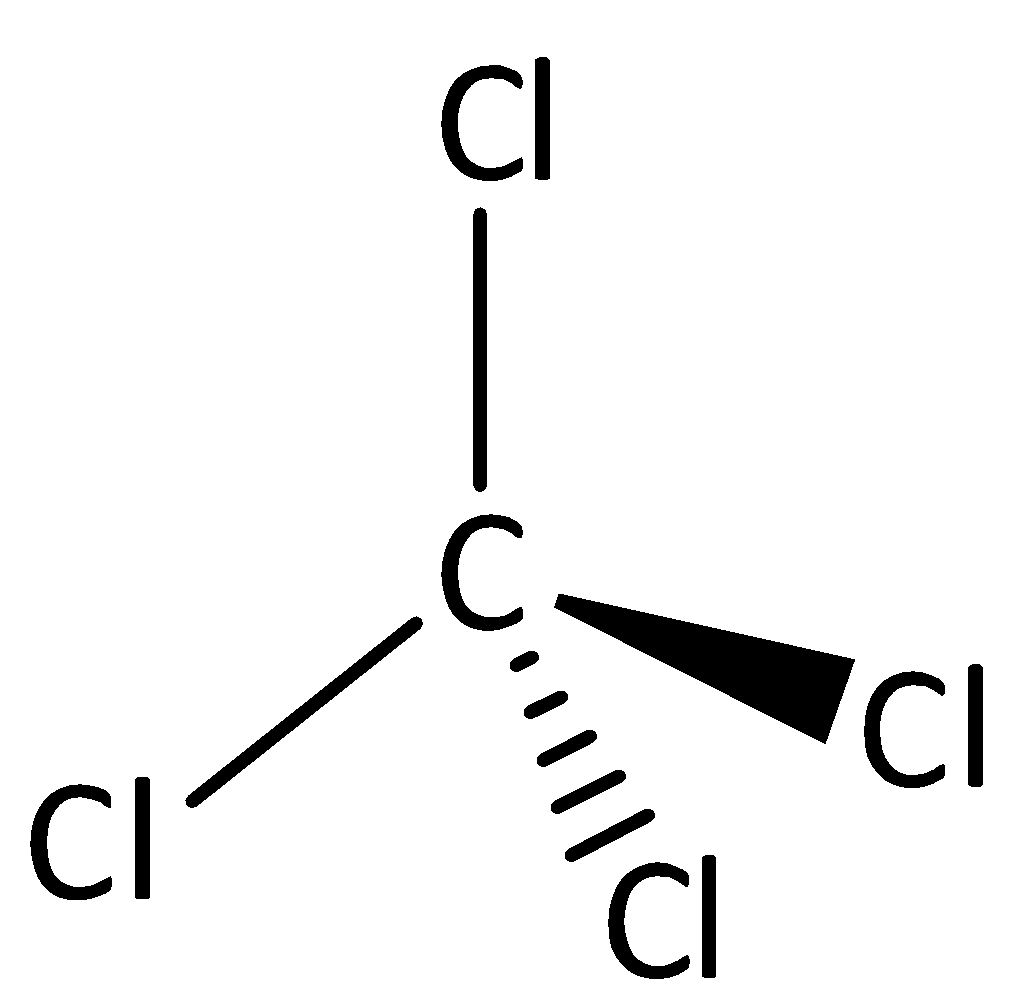
Carbon tetrachloride is a tetrahedral molecule with fluorine atom type, the net dipole moment of the molecule is zero. Therefore, the option D is incorrect.
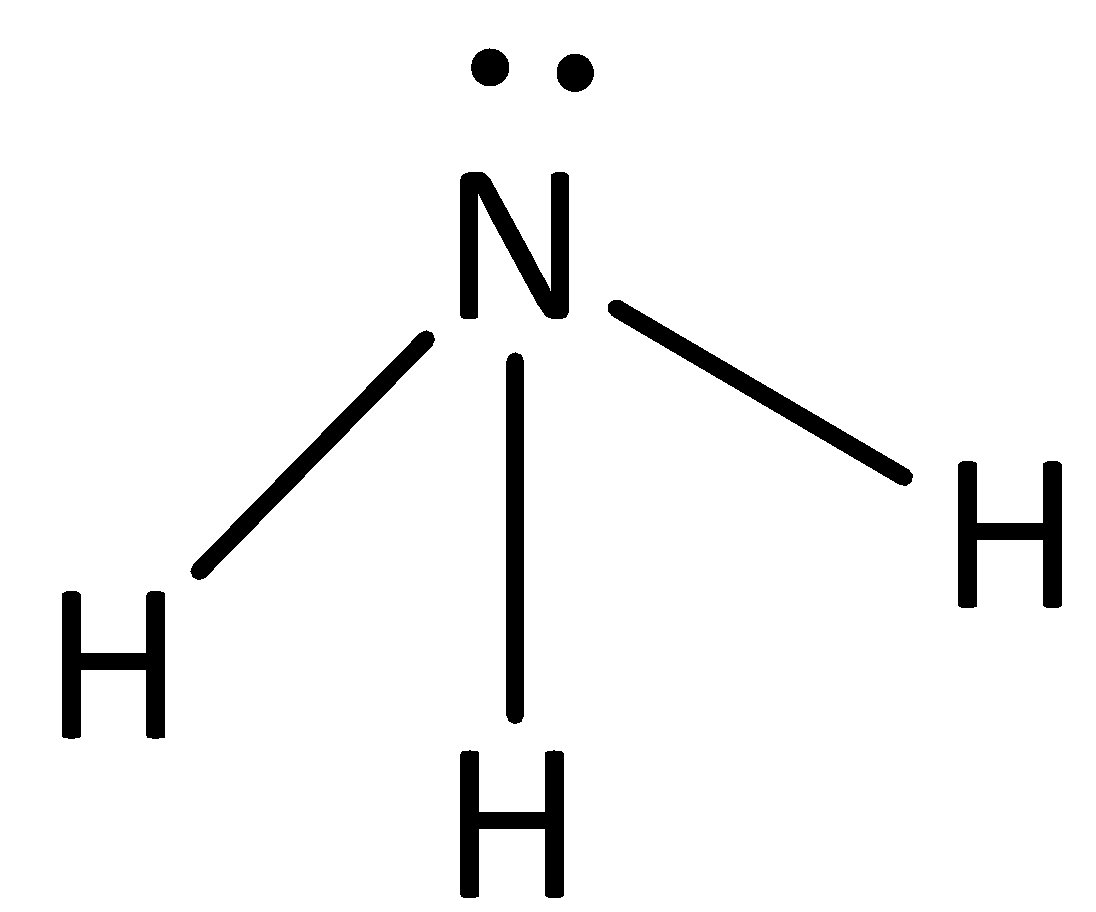
The structure of ammonia is,
The structure of NF3 is,
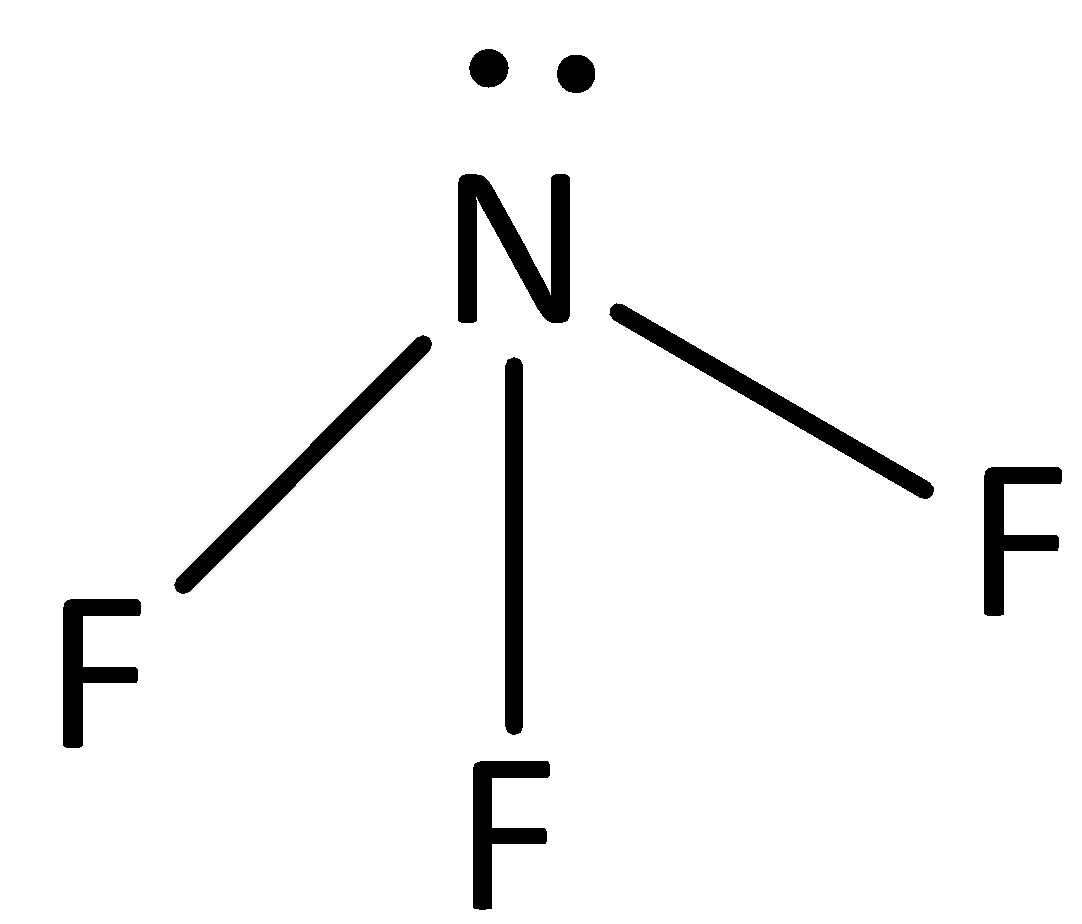
The dipole moment of N - H bond is less than that of N - F bond as the fluorine atom is more electronegative than nitrogen and it attracts the bonding electrons to larger extent. The overall dipole moment of ammonia is lesser than NF3, whereas in ammonia, the charge separation is less than NF3 molecule.
Therefore, the option A is correct.
Note:
We know that polarity results from the uneven partial charge distribution between various atoms in a compound. Atoms, like nitrogen, oxygen, and halogens that are more electronegative tend to have partial negative charges. Atoms, like carbon and hydrogen, tend to be more neutral or have partial positive charges.
The polar nature of the molecules can be measured by the dipole moment. A molecule with zero dipole moment then it is a nonpolar molecule. If a molecule has a net dipole moment then it is a polar molecule.
