Question
Question: The dipole moment of \(N{F_3}\) is less than \(N{H_3}\) because of the following: A.\(N{H_3}\) for...
The dipole moment of NF3 is less than NH3 because of the following:
A.NH3 forms associated molecules
B.F is more electronegative than N
C.The resultant bond polarity is less
D.The resultant of individual polarities that is opposed by polarity of lone pairs in NF3 .
Solution
Dipole moment is defined as the difference in electronegativity between two chemically bonded atoms.it can arise in any of the system when there is a separation of charge. It is denoted by the symbol ′d′ .
Complete step by step answer:
-Dipole moment occurs when there is a difference in electronegativity between two chemically bonded atoms.
-It is commonly denoted by the symbol (D) .
-It is also denoted as ′μ′
-The formula of dipole moment is as follows:
Dipole Moment=charge×distance separation
μ=Q×r .
-Dipole moment can be measured in Debye units D .
The value of Debye unit is D=3.33564×10−30C.m.
Where C=Coulomb, m=meter .
-Properties of NF3 and NH3 are as follows:
-Structure of both the molecules is pyramidal.
-In NF3 , fluorine has the tendency to accept electrons whereas in NH3 , hydrogen is comparatively small and can easily donate its electron.
-Both the molecules have lone pairs of electrons and three bond pairs.
-Also they both are inorganic compounds.
Now, we will see the structure of NF3 and NH3 as follows:
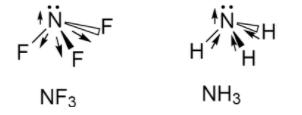
The dipole moment of NF3 is 0.24D whereas the dipole moment of NH3 is 1.46D .
In the case of NH3 ,since the nitrogen is more electronegative than hydrogen, so it will try to pull the electrons from hydrogen atoms thus the polarity will be towards the nitrogen atom.
In the case of NF3 ,since the fluorine being more electronegative than nitrogen will try to pull the electrons from nitrogen opposite from that of nitrogen, so the polarity is towards the fluorine.
So, the correct answer is option D) The resultant of individual polarities is opposed by the polarity of the lone pair in NF3 .
Note:
In this dipole moment helps us to know the electronegativity of an element because if the dipole moment is greater than it means that electronegativity will be greater. Even by electronegativity we can guess whether the molecule has less or more dipole moment.
