Question
Question: The diagram shows the melting and boiling points of four different substances. Which substance has a...
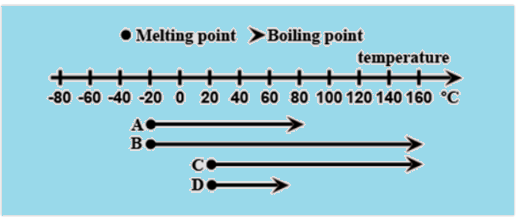
The diagram shows the melting and boiling points of four different substances. Which substance has a melting point at −20∘C and a boiling point at 160∘C ? Also identify the two substances having the same boiling points.
Solution
In this question, we can find the melting point and boiling point of each substance separately. After that we can find the substance for the required melting point and boiling point. It is possible that more than one substance has the same melting point or same boiling point.
Complete step by step solution:
Let us make a table for melting points of the substances and the boiling points of the substances according to the diagram.
| Substance | Melting point∘C | Boiling point∘C |
|---|---|---|
| A | −20 | 80 |
| B | −20 | 160 |
| C | 20 | 160 |
| D | 20 | 70 |
According to the above table,
Substance A and B have the melting point −20∘C. Substance B and C have the boiling point 160∘C.
So, substance B has the melting point −20∘C and boiling point 160∘C.
Now, again from the above table,
Substance B and C have the boiling point 160∘C.
Additional Information:
Temperature- Temperature can be defined as a physical quantity that expresses hot or cold. We can differentiate an object whether it is cold or hot by temperature.
Melting Point- It is defined as a temperature where the solid phase and liquid phase are in equilibrium.
Boiling Point- It is defined as a temperature at which the vapour pressure of a liquid is equal to the external pressure i.e. surroundings pressure.
Note: It is possible that two substances may have the same melting point and same boiling point. It is also possible that two substances may have only the same melting point. It is also possible that two substances may have only the same boiling point.
