Question
Question: The diagram shows a diaphragm cell used for the electrolysis of brine. Brine is concentrated aqueous...
The diagram shows a diaphragm cell used for the electrolysis of brine. Brine is concentrated aqueous sodium chloride. A solution of sodium chlorate (I) which is commonly used as bleach, can be made by mixing which of the two substances?
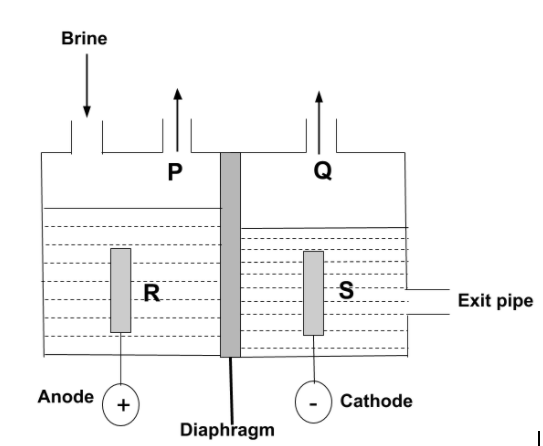
A.P and R
B.P and S
C.Q and R
D.Q and S
Solution
The chemical formula for sodium chlorate is NaClO3 which can be formed by the reaction between chloride and sodium hydroxide. Brine is a high concentration solution of sodium chloride salt in the water.
Complete step by step answer:
-The high concentration solution of salt sodium chloride NaCl in water, H2O is called Brine. Lower levels of concentration can be called by other names: saline water, freshwater and brackish water.
-Sodium chlorate is the white crystalline powder which is readily soluble in water and is the inorganic compound with the molecular formula, NaClO3 .
-Now, according to the question, the electrolysis of brine is carried out. So, we have to find which substances are used for the formation of sodium chlorate, NaClO3. In this electrolysis, the diaphragm cell process is used in which there are two compartments separated by permeable diaphragm which is generally made of asbestos fibers. Brine is introduced into the anode and flows into the cathode compartment.
-The chloride ion is negatively charged, so it can get attracted towards positively charged anode. So, P can be Cl2 , whereas sodium ions and hydrogen ions from the water are positively charged so, they get attracted towards negative cathode, then, the dilute solution of sodium hydroxide is produced at cathode, so, S can be NaOH. Hence, the reaction is –
6NaOH+3Cl2NaCl3+5NaCl+3H2O
-Therefore, the sodium chlorate solution can be made by mixing P and S.
Hence, the correct option is (B).
Note:
When the electrolysis of aqueous NaCl is carried out we have to take water into equation. Water can be oxidized and reduced both and it competes with the dissolved Na+ and Cl− ions. This produces hydrogen instead of producing sodium.
