Question
Question: The depression in freezing point of 0.01 molal aqueous CH3COOH solution is 0.02046°C. 1 molal urea s...
The depression in freezing point of 0.01 molal aqueous CH3COOH solution is 0.02046°C. 1 molal urea solution freezes at -1.86°C. Assuming molality is equal to molarity, pH of the solution would be
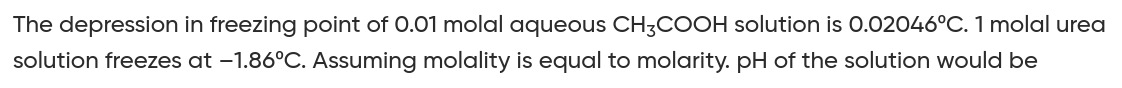
2
3
4
5
3
Solution
1. Calculate the cryoscopic constant (Kf) for water:
Urea is a non-electrolyte, so its van't Hoff factor (i) is 1. The freezing point depression for a 1 molal urea solution is ΔTf=0∘C−(−1.86∘C)=1.86∘C. Using the formula ΔTf=i⋅Kf⋅m:
1.86∘C=1⋅Kf⋅1 molal
Kf=1.86 K kg mol−1
2. Calculate the van't Hoff factor (i) for the CH3COOH solution:
For the 0.01 molal CH3COOH solution, the depression in freezing point (ΔTf) is 0.02046°C. Using the formula ΔTf=i⋅Kf⋅m:
0.02046=i⋅1.86⋅0.01
i=1.86⋅0.010.02046=0.01860.02046
i=1.1
3. Determine the degree of dissociation (α) for CH3COOH:
Acetic acid (CH3COOH) is a weak acid that dissociates as:
CH3COOH⇌H++CH3COO−
It dissociates into 2 ions (n=2). The relationship between the van't Hoff factor (i) and the degree of dissociation (α) is:
i=1+(n−1)α
1.1=1+(2−1)α
1.1=1+α
α=1.1−1=0.1
4. Calculate the concentration of H+ ions:
The initial concentration of CH3COOH (C) is 0.01 molal. Assuming molality is equal to molarity, C=0.01 M. The concentration of H+ ions at equilibrium is given by:
[H+]=Cα
[H+]=0.01 M×0.1
[H+]=0.001 M
[H+]=10−3 M
5. Calculate the pH of the solution:
pH=−log[H+]
pH=−log(10−3)
pH=3
