Question
Question: The decreasing order of stability of following cation is ...
The decreasing order of stability of following cation is
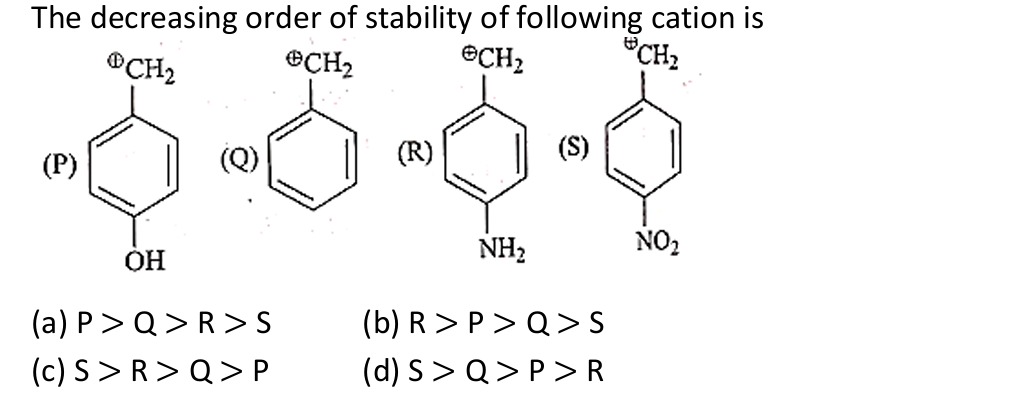
P > Q > R >S
R > P > Q >S
S > R > Q >P
S > Q > P >R
R > P > Q >S
Solution
The stability of a carbocation is increased by electron-donating groups and decreased by electron-withdrawing groups. These effects can be inductive or resonance effects. For substituents on an aromatic ring, resonance effects (+R or -R) are often more significant than inductive effects (+I or -I), especially when the charge is adjacent to the ring.
Let's analyze the effect of the substituent at the para position on the stability of the benzylic carbocation.
(P) Para-hydroxybenzyl carbocation: The -OH group is electron-donating by resonance (+R effect) and electron-withdrawing by induction (-I effect). The +R effect is stronger than the -I effect, making -OH an overall electron-donating group. The resonance donation from the oxygen's lone pair stabilizes the positive charge by delocalizing it into the ring and onto the oxygen atom, forming a resonance structure with a positive charge on oxygen and a double bond between the ring and oxygen.
(Q) Benzyl carbocation: This is the parent carbocation, stabilized by resonance with the benzene ring.
(R) Para-aminobenzyl carbocation: The -NH2 group is strongly electron-donating by resonance (+R effect) and weakly electron-withdrawing by induction (-I effect). The +R effect is much stronger than the -I effect, making -NH2 a strong overall electron-donating group. The resonance donation from the nitrogen's lone pair is more effective than that from oxygen due to the lower electronegativity of nitrogen, leading to a more stable resonance structure with a positive charge on nitrogen and a double bond between the ring and nitrogen.
(S) Para-nitrobenzyl carbocation: The -NO2 group is strongly electron-withdrawing by resonance (-R effect) and also electron-withdrawing by induction (-I effect). Both effects destabilize the carbocation by withdrawing electron density from the benzene ring, which makes it harder to delocalize the positive charge from the benzylic carbon into the ring.
Comparing the effects: -NH2 is a stronger electron-donating group (+R) than -OH (+R). -NO2 is a strong electron-withdrawing group (-R and -I). The unsubstituted case (Q) has no significant electron-donating or withdrawing group.
The stability order of carbocations is: Strong electron-donating groups > Weak electron-donating groups > No substituent > Electron-withdrawing groups.
Based on the strengths of the substituents: -NH2 (strong +R) is the most stabilizing. -OH (moderate +R) is less stabilizing than -NH2. H (in Q) is the reference. -NO2 (strong -R and -I) is the most destabilizing.
Therefore, the decreasing order of stability is R > P > Q > S.
