Question
Question: The decreasing order of basic nature in the following is: i.Pyrrole ii.Pyridine iii.Aniline A. i...
The decreasing order of basic nature in the following is:
i.Pyrrole ii.Pyridine iii.Aniline
A. ii > iii > i
B. ii > i > iii
C. iii > ii > i
D. iii > i > ii
Solution
Whenever a question asks about the comparison of basic characters of compounds, check the comparison about the protonation of compounds. The compounds that are easily protonated or have protonated electrons or negative charge are said to be more basic in nature.
Complete answer:
In order to answer our question, we need to learn about the factors that affect basic strength of a compound. Now, bases are known to be those compounds that are easily protonated. They carry excess electrons on them and also carry a negative charge. Apart from these, there are other factors that determine the basic strength. Electron donating groups exert +I effect and increase the concentration of electrons. Stability also plays a role in determining the basic character. A compound which is more stable, or rather, delocalised is said to be more basic. This is achieved by +R groups. Let us look at the structures:
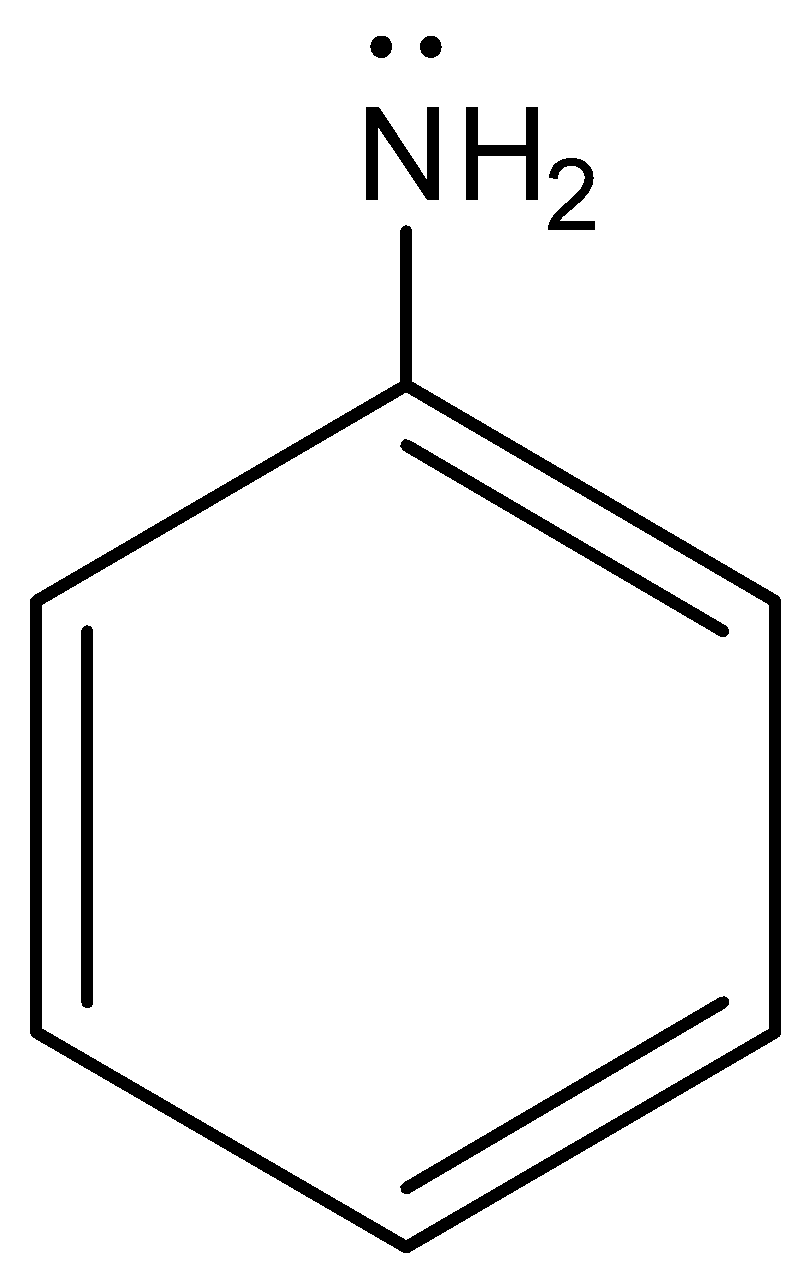
This is the structure of aniline and we can see that the nitrogen has a lone pair of electrons that participate in protonation.
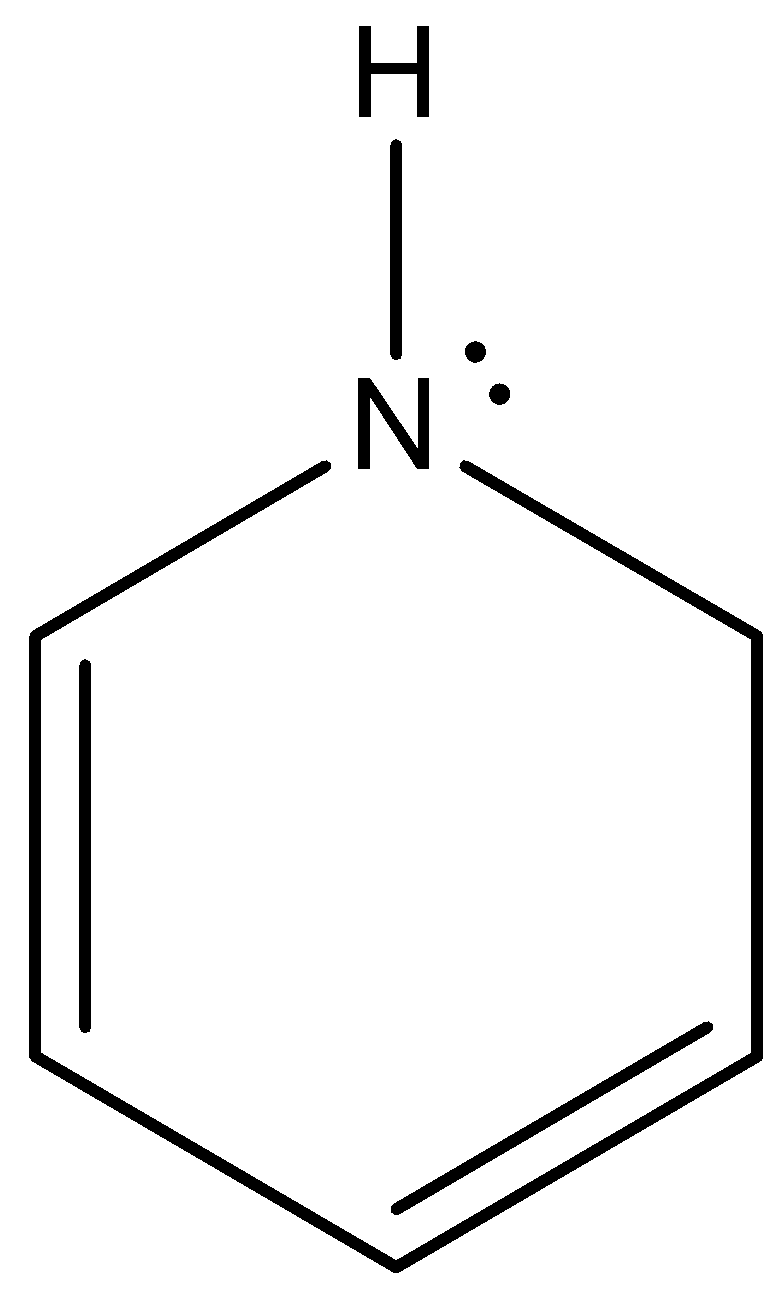
This is the structure of pyrrole and although nitrogen has a lone pair of electrons, those are not involved in protonation as there is resonance.
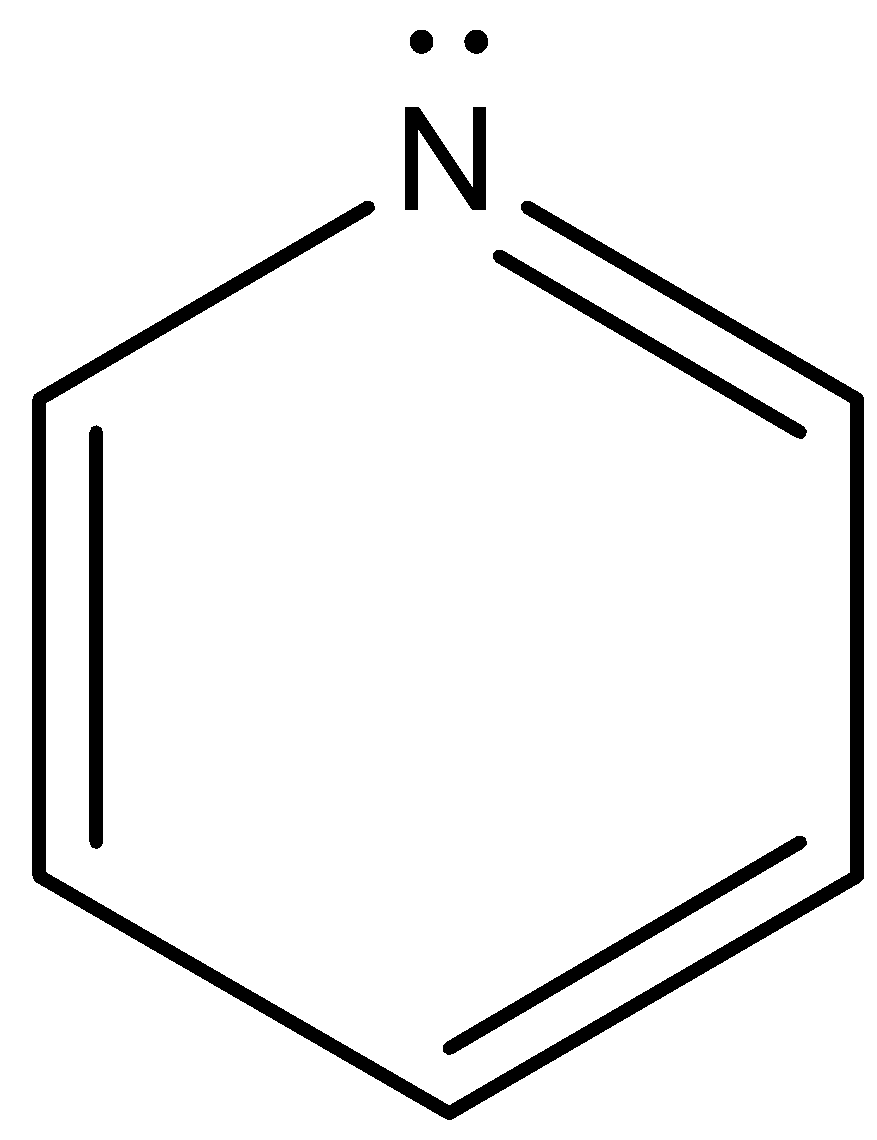
This is the structure of pyridine and as we can see, the lone pair of electrons on the nitrogen, although involved in resonance, can participate in protonation, but not much.
So, comparing the three compounds, aniline has the maximum basic strength, followed by pyridine and pyrrole.
So we get our answer as option C.
Note:
In case our question had a compound , then this compound 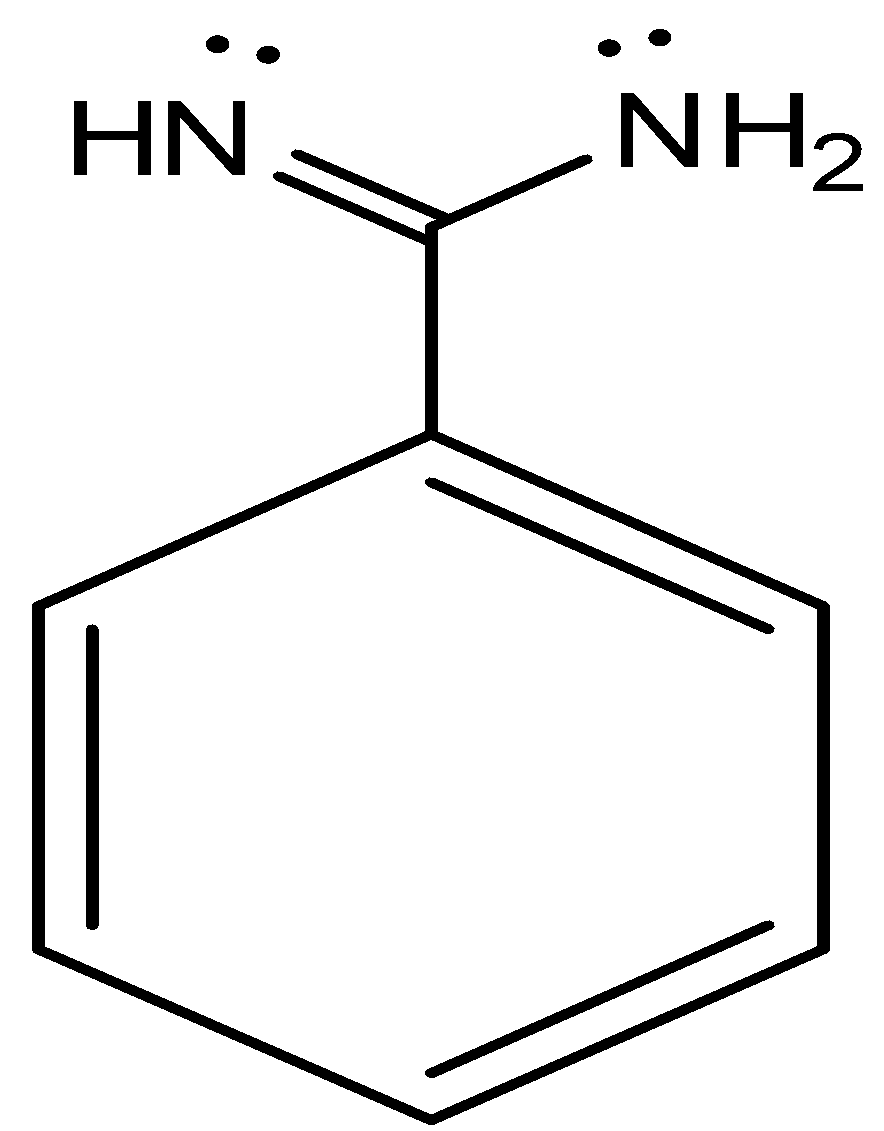 would have had the maximum basic strength, as there are 2 nitrogens in this compound and each nitrogen has lone pair for protonation. It is stable too.
would have had the maximum basic strength, as there are 2 nitrogens in this compound and each nitrogen has lone pair for protonation. It is stable too.
