Question
Question: The d-orbitals involved in \[s{{p}^{3}}{{d}^{2}}\] or \[{{d}^{2}}s{{p}^{3}}\] hybridization of the c...
The d-orbitals involved in sp3d2 or d2sp3 hybridization of the central metal ion are:
(this question has multiple correct options)
a.) dx2−y2
b.) dxy
c.) dyz
d.) dz2
Solution
From the given hybridization we can say that the geometry of the compound has octahedral. So, to solve this question, consider the splitting of d-orbitals in an octahedral complex, when approached by a ligand.
Complete step by step solution:
When any negatively charged species or ligand approaches a metal, the energy of the orbitals increases. This leads to the splitting of orbitals as –
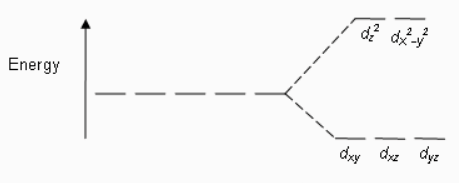
In octahedral complexes, the splitting of orbitals always takes place in this manner.
The electron first goes to the lower energy orbital, i.e. dxy.dyz,dz2and then to the higher energy orbital, i.e., dx2−y2and dz2. Therefore, bonding electrons go to the high energy orbitals which decide the hybridization of the compound.
In case of sp3d2 hybridization, nd (outermost) orbitals are involved. The compound is therefore also known as outer-orbital complex.
In case of d2sp3 hybridization, (n-1)d (next to outermost) orbitals are involved. The compound is therefore also known as inner-orbital complex.
Therefore, the answer is – option (a) and option (d).
Additional information: In short, if inner d-orbital is used for bonding, the hybridization will be d2sp3. Similarly, if outer d-orbital is used, the hybridization will be sp3d2.
Note: Irrespective of the differences between sp3d2 and d2sp3, there are certain similarities, such as –
Both hybridization results in octahedral geometry.
Both have six hybrid orbitals.
There is an angle of 90 degrees between hybrid orbitals.
