Question
Question: The d-orbital involved in \[s{p^3}d\] hybridization is: A. \[{d_{{x^2} - {y^2}}}\] B. \[{d_{xy...
The d-orbital involved in sp3d hybridization is:
A. dx2−y2
B. dxy
C. dz2
D. dzz
Solution
To determine the hybridization of any compound, we must know the structure of that compound. Hybridization is defined as a concept of mixing two atomic orbitals having the same energy levels to give new degenerate orbitals.
Complete step by step answer:
The formula to calculate the hybridization of the central atom of the molecule is, H=21[V+X−C+A] . where V is the number of valence electrons of the central atom, X is the number of monovalent atoms attached to the central atom, C is the total cationic charge and A is the total anionic charge, H is the hybridization.
| Hybridization number(H) | Hybridization | geometry |
|---|---|---|
| 2 | Sp | linear |
| 3 | sp2 | Trigonal planar |
| 4 | sp3 | tetrahedral |
| 5 | sp3d | Trigonal bipyramidal |
| 6 | sp3d2 | octahedral |
| 7 | sp3d3 | Pentagonal bipyramidal |
For, sp3d hybridization, the hybridization number (H) should be 5. These five orbitals are degenerate, that is their energies are the same but configurations are different.
In this sp3d hybridization there are a total of 5 orbitals which are, s, Px , Py , and Pz . In the case of d subshell, the dz2 orbital takes part in this hybridization.
So, the correct answer is C.
Additional information:
For, SF4 the hybridization is,
For H=5 , the hybridization would be sp3d , so the geometry of SF4 is Trigonal bipyramidal. In this case, the central atom chlorine has 4 bond pairs and 1 lone pair, therefore, the shape is a see-saw. Due to this shape, it has a permanent dipole moment. The structure is shown below,
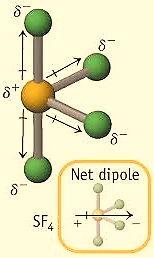
Remember that when a lone pair is present, then the molecular geometry will be the three-dimensional arrangement of the atoms without the lone pair. If there is no lone pair only bond pairs then the number of bond pairs can be equal to the hybridization number.
Note: In the case of sp3d2 hybridization there are a total of 6 orbitals which are, s, Px , Py , and Pz . In the case of d subshell, the dz2 and dx2−y2 orbitals take part in this hybridization. In the case of sp3d3 hybridization there is a total of 7 orbitals which are, s, Px , Py , and Pz . In the case of d subshell, the dz2 , dxy and dx2−y2 orbitals take part in this hybridization.
