Question
Question: The d-electron configurations of \[{\text{C}}{{\text{r}}^{{\text{2 + }}}}{\text{,M}}{{\text{n}}^{{\t...
The d-electron configurations of Cr2 + ,Mn2 + ,Fe2 + and Co2 + are d4,d5,d6 and d7respectively. Which one of the following will exhibit minimum paramagnetic behaviour?
(Atomic numbers of Cr = 24, Mn = 25, Fe = 26, Co =27)
A.[Mn(H2O)6]2 +
B.[Fe(H2O)6]2 +
C.[Co(H2O)6]2 +
D.[Cr(H2O)6]2 +
Solution
Water is a strong ligand thus no pairing of electrons takes place while filling of electrons in orbitals. The presence of unpaired electrons leads to paramagnetic behaviour.
Complete step by step answer:
In all the given complexes, the oxidation state of metals such as Cr,Mn,Fe and Co is +2 thus the outer shell electronic configuration becomes d4,d5,d6 and d7 respectively. The 3d orbital diagram for each complex can be depicted as below:
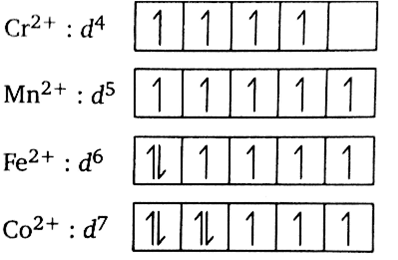
The unshared pair of electrons simply refers to the pair of valence electrons which are not shared during a covalent bonding. This term is sometimes called a lone pair of electrons or non-bonding pair of electrons. The presence of unpaired electrons indicates the paramagnetic behaviour. Greater the number of electrons greater the paramagnetic behaviour. Magnetic moment can be calculated from the spin only formula,
μ=n(n + 2) BM (BM- Bohr magneton, unit of magnetic moment)
where n is the number of unpaired electrons. More the unpaired electrons more the magnetic moment.
In the given complexes, [Mn(H2O)6]2 + has the maximum number of unpaired electrons (5 unpaired electrons) thus that complex will exhibit maximum paramagnetic behaviour.
While [Co(H2O)6]2 + has the minimum number of unpaired electrons (3 unpaired electrons) thus that complex will exhibit minimum paramagnetic behaviour.
So, the correct option is C.
Note:
In case of absence of unpaired electrons, there will be no paramagnetic behaviour instead the complex will exhibit diamagnetic behaviour.
