Question
Question: The crystalline structure of \[NaCl\] is A. Hexagonal close packing B. Face-centred cubic C. ...
The crystalline structure of NaCl is
A. Hexagonal close packing
B. Face-centred cubic
C. square planar
D. body-centred cubic
Solution
A unit cell that has a lattice point at every face centre in addition to the lattice point at every corner is called a face-centred cubic unit cell. In the case of NaCl, the ratio of chloride to the ratio of sodium ions is 1:1.
Complete step by step answer:
In the case of NaCl, the structure is a rock salt type structure with coordination number being in a ratio of 6:6 which means that there are 6 chloride ions present on the 6 faces of the face centred cubic system and 6 Na+ ions occupy the octahedral voids in the lattice system. There are many compounds that have a similar structure as the sodium chloride structure. The ratio of radius range of anions and cations ranges between 225-414 nm. Those structures which have a similar structure to that of NaCl include different alkyl halides, oxides of different metals, etc. In the case of a face-centred cubic system, the coordination number will be 12 and the distance between the two nearest atoms will be 2a2 . The relation between the radius and side length in such a system is r=22a .
Thus, the correct option is (B) Face-centred cubic.
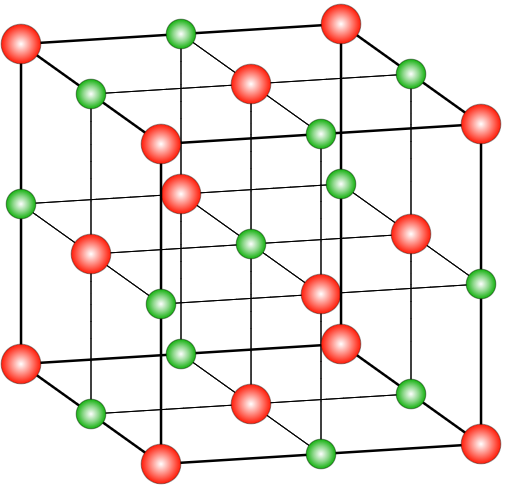
where red spheres represent Cl− and green ones are for Na+
Note:
In a face centred cubic system, along the face diagonal, all the atoms touch each other and the length of the face diagonal is a2 . The number of atoms per unit cell in FCC lattice is 4 and the packing efficiency is 74% with void space of 26%.
