Question
Question: The correct structure of ethylenediaminetetraacetic acid (EDTA) is  is
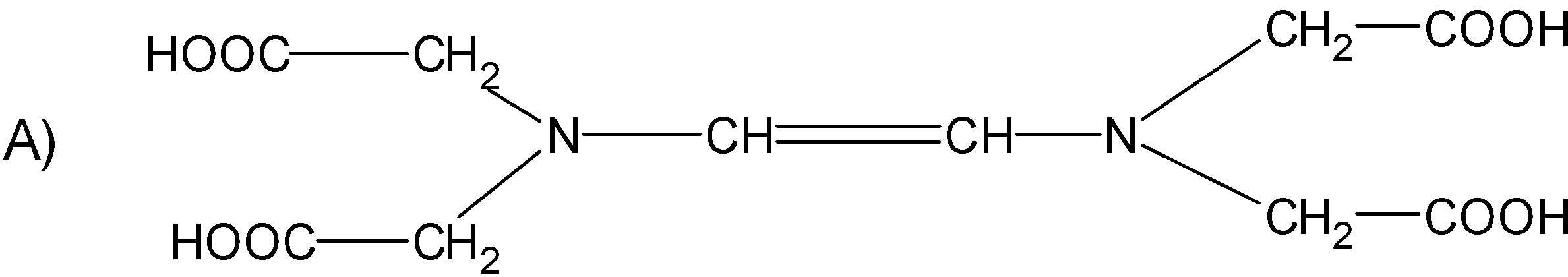
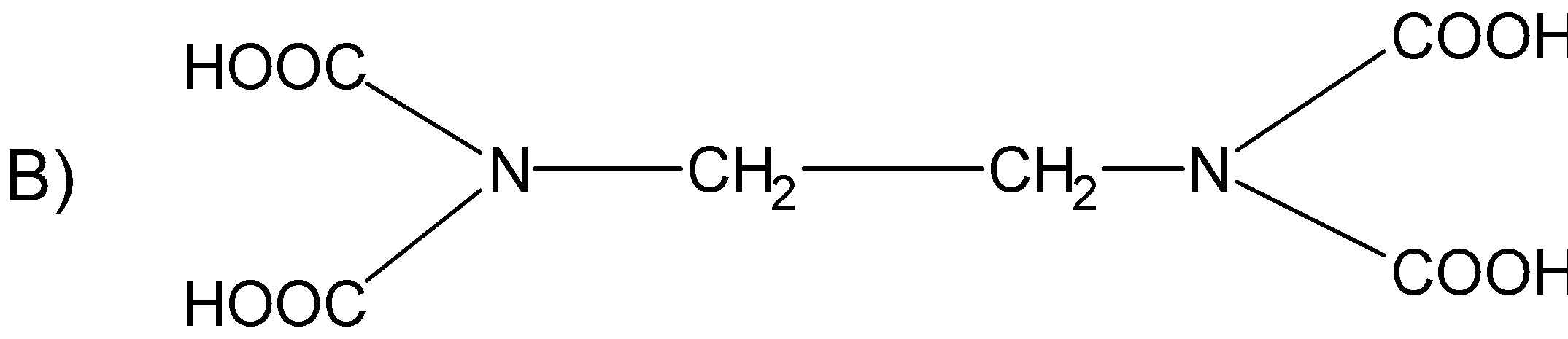
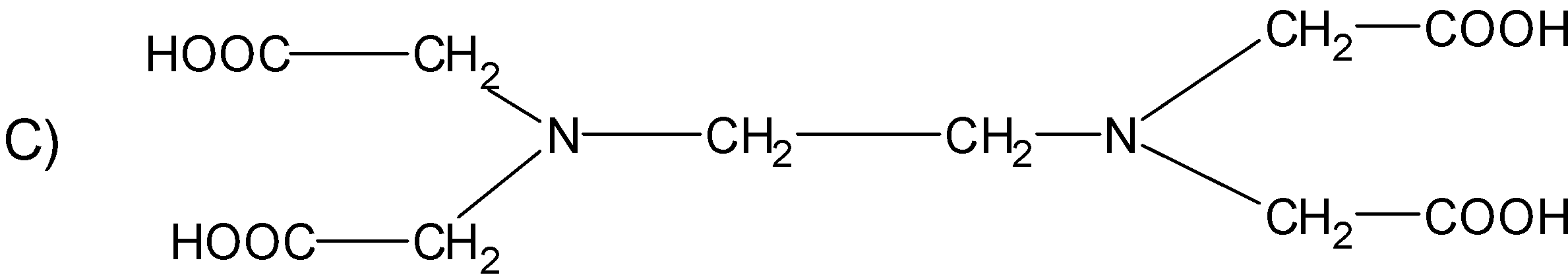
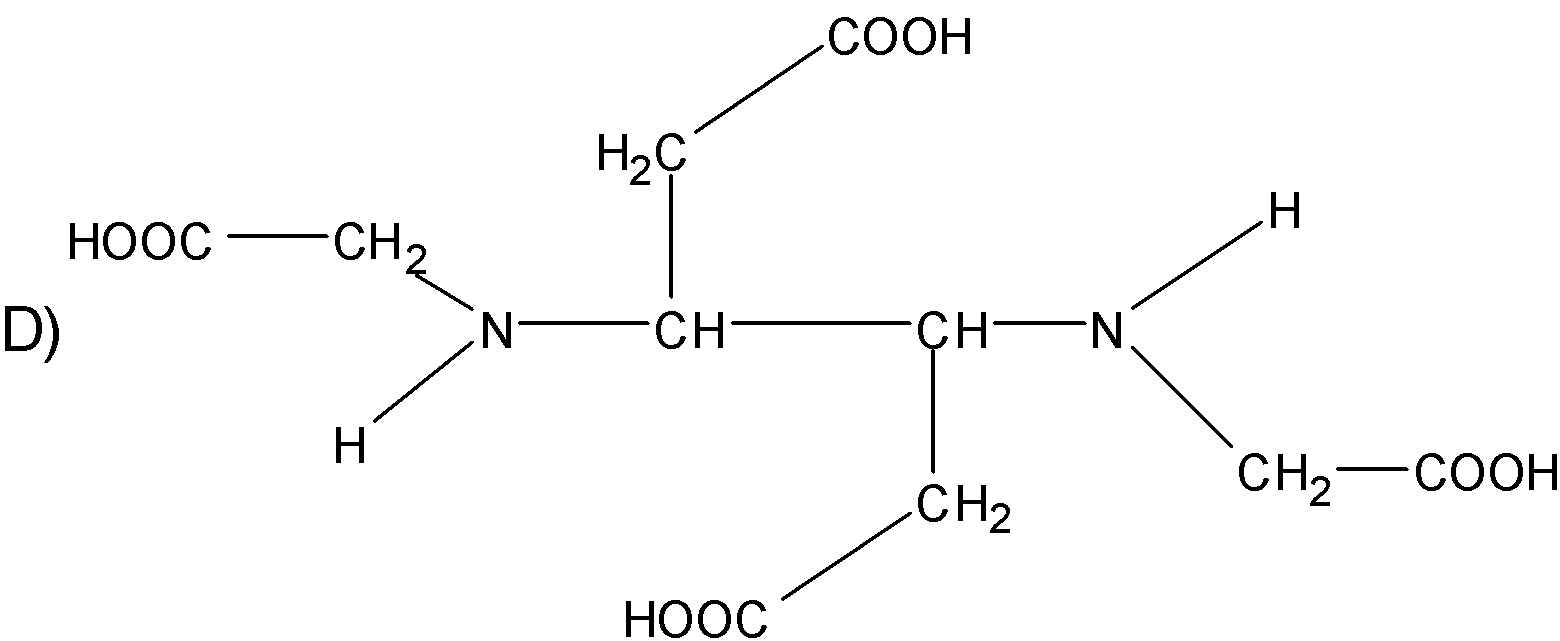
Solution
Ethylene diamine tetra acetic acid or the EDTA is a tetraprotic acid. The structure of it consists of the ethane with two amine groups attached to it forming an ethylene diamine. Each of the hydrogen on the amine group is replaced by the acetic acid (-CH2COOH) group. The resultant structures are theEDTA. It is a complexing agent.
Complete answer:
Ethylene diamine tetra acetic acid or EDTA is a complexing agent.
EDTA Was first synthesized by Ferdinand Munz in 1935. This EDTA is also called the amino polycarboxylic acid. It is colourless and water soluble in its salt form.
The structure EDTA consists of the basic ethane group which is attached with the two -NH2 groups at the terminal positions forming an ethylene diamine. The two hydrogens from each amine group situated at the terminal positions are replaced by the acetic acid group(-CH2COO). There are a total of four acetic acid groups attached to the ethylene diamine.
The structure for the ethylene diamine tetra acetic acid or EDTA is as follows.
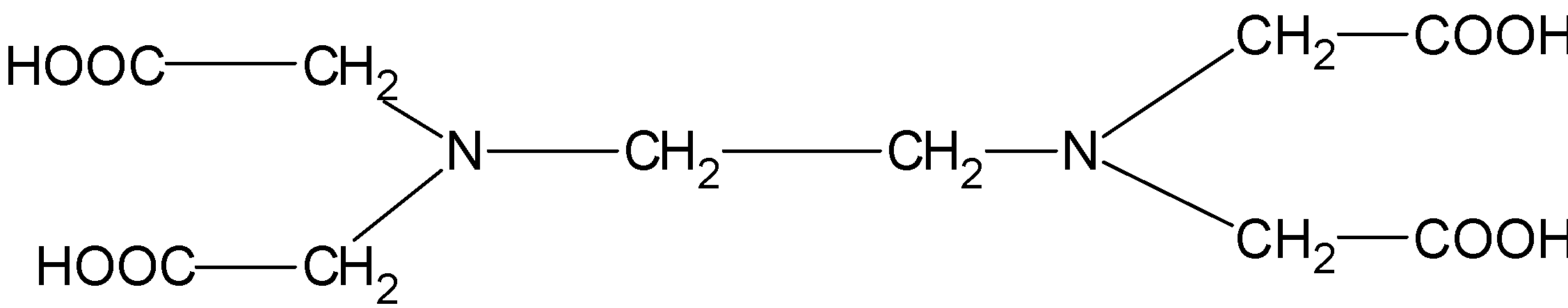
EDTA is a hexadentate ligand.
It has four carboxylic acid groups and two amine groups with lone pairs of electrons on it. It is polyprotic acid.
Along with the four carboxylic acid groups EDTA can add two more hydrogens on the two nitrogens of the amine groups.
Hence, (C) is the correct option.
Additional information:
The EDTA has the special ability to form a complex with the metals. It forms the 1:1metal-EDTA complexes. EDTA Binds with the metal ions to form a soluble complex of metal.
As EDTA can donate four electrons the complex formed by the metal and EDTA is as shown below:
M+n+Y-4→MYn-4
Where M is the metal and y is the complexing agent EDTA.
Metal analysis can be done using the titration of EDTA the metal using the metal ion indicators. One of the famous and most common uses of EDTAis to determine the hardness of the water.
Ca2++Na2-EDTA⇄Ca-EDTA+2Na+
It is usually present as a disodium salt of EDTA which is readily soluble in water.
Note:
EDTAis an abbreviation used for the Ethylene Diamine Tetra-Acetic acid. It is acid thus in the acidic medium it is fully protonated. Hence most of the metal-EDTA reactions favours in slightly basic or basic conditions.
