Question
Question: The correct statement (s) related to allotropes of carbon is/are: This question has multiple corre...
The correct statement (s) related to allotropes of carbon is/are:
This question has multiple correct options
A. Graphite is the most stable allotropes of carbon and has a two dimensional sheet like structure of hexagonal rings of carbon.
B. diamond is the hardest allotrope of carbon and having a three dimensional network structure of C(sp3)
C.fullerene (C60) is recently discovered non-crystalline allotrope of carbon having a football like structure
D.van der waals force of attraction acts between the layers of graphite 6.14A away from each other.
Solution
We know that, a property by virtue of which some elements exist in two or more forms possessing the same physical state. Many elements such as carbon, sulphur have allotropes. Carbon has three allotropes namely, fullerene, graphite and diamond.
Complete step by step answer:
Let’s identify the correct answers from the options.
Option A says that graphite is the most stable allotrope and possesses 2D sheets like the structure of hexagonal carbon rings. The structure of graphite is shown below:
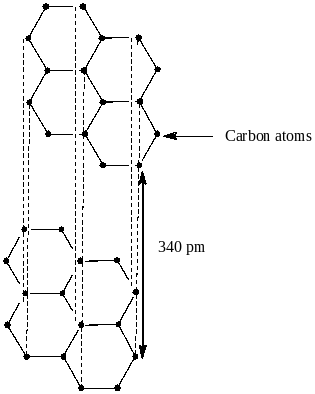
We see from the above structure that graphite has two dimensional sheets like hexagonal carbon rings. Also it has delocalized π electrons over the entire sheet. The result of delocalization is lowering of energy of the system which results in greater stability of graphite. So, option A is correct.
Option B says diamond is the hardest allotrope of carbon and it has a three dimensional network structure of C(sp3).
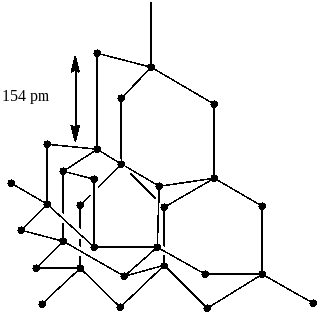
In diamond, every carbon atom possesses sp3 hybridization and is linked to other four carbon atoms using the hybridized orbitals in tetrahedral geometry. The breaking of extended covalent bonding is very difficult and therefore, the hardest substance in the Earth is diamond. Also it has an SD network. So, option B is also correct.
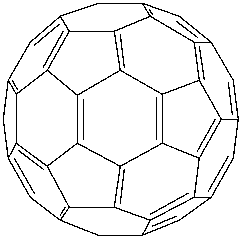
Option C says fullerene (C60) is a recently discovered non-crystalline allotrope of carbon possessing a football-like structure. Fullerene is only the pure form of carbon as it has smooth structure with no dangling bonds. Its structure is like a football and was discovered in 1970. So, it was not discovered. So, option C is not correct.
Option D says van der waals force of attraction acts between the layers of graphite 6.14A away from each other
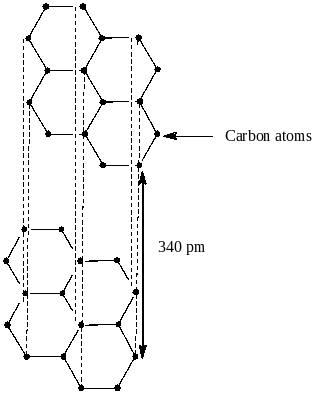
From the structure, we clearly see that the distance between the layers of graphite is 340 pm or 3.4A0. So, option D is also not correct.
Hence, the correct answer is option A and B.
Note:
Diamond is the hardest allotrope of carbon and it is used in drilling, cutting. It does not undergo reaction with any chemical reagents including strong base or acids. The diamond surface is hydrophobic and lipophilic, that means, it cannot get by water.
