Question
Question: The correct statement(s) regarding the following are: (i) \(HClO\) (ii) \(HCl{{O}_{2}}\) (iii) \(H...
The correct statement(s) regarding the following are:
(i) HClO (ii) HClO2 (iii) HClO3 (iv) HClO4
(A) the number of Cl=O bonds in (ii) and (iii) together is three
(B) the number of lone pairs of electrons on Cl in (ii) and (iii) together is three
(C) the hybridization of ClO in (iv) is sp3
(D) amongst (i) to (iv), the strongest acid is (i)
Solution
Draw the structure of the molecules. Determine the number of double bonds present in them. Also, determine the number of lone pairs on chlorine atoms in each structure. Find out the oxidation states of Cl atoms in each structure.
Complete answer:
Chlorine forms four types of oxyacids:
-Hypohalous acid: Hypochlorous acid
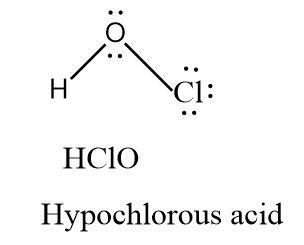
Hypochlorous acid has one oxygen atom containing two lone pairs of electrons and one chlorine atom containing three lone pairs of electrons. There are no double bonds present in the molecule.
Oxidation state = +1
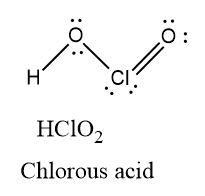
Chlorous acid has two oxygen atoms containing two lone pairs of electrons each and one chlorine atom containing two lone pairs of electrons. There is one Cl=O double bond present in the molecule.
Oxidation state = +3
-Halic acid: Chloric acid
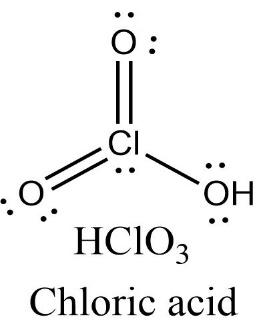
Chloric acid has three oxygen atoms containing two lone pairs of electrons each and one chlorine atom containing one lone pair of electrons. There are two Cl=O double bonds present in the molecule.
Oxidation state = +3
-Perhalic acid: Perchloric acid
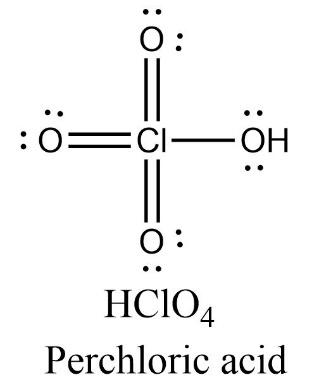
Perchloric acid has four oxygen atoms containing two lone pairs of electrons each and one chlorine atom containing no lone pair of electrons. There are three Cl=O double bonds present in the molecule.
Oxidation state = +5
Now, let’s take a look at the options given in the question.
(A) It is correct because the number of Cl=O bonds in HClO2 and HClO3 together is three.
(B) It is correct because the number of lone pairs of electrons in HClO2 and HClO3 together is three.
(C) It is incorrect because the hybridization of Cl=O in HClO4 is sp2.
(D) We know that as the oxidation state increases, acidity increases. In this case, the oxidation state of Perchloric acid is +7. So, Perchloric acid is the strongest acid amongst other oxyacids of chlorine.
Order of Acidity can be given as,
HClO4>HClO3>HClO2>HClO
So, we can conclude that option (D) is also incorrect because it states hypochlorous acid is the strongest acid.
So, the correct answer is “Option A and B”.
Note: Mistakes can happen while drawing the structure of molecules. Remember, a doubly bonded oxygen atom has two lone pairs and a singly bonded chlorine atom has three lone pairs.
