Question
Chemistry Question on p -Block Elements
The correct statement(s) about the oxoacids, HClO4 and HClO, is(are)
A
The conjugate base of HClO4 is weaker base than H2O
B
The central atom in both HClO4 and HClO is sp3 hybridized.
C
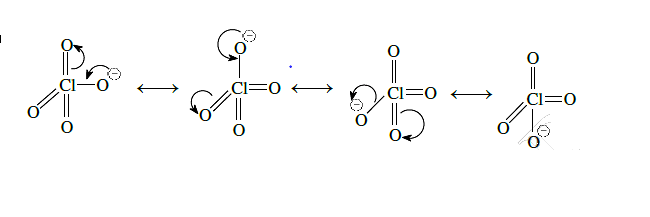
HClO4 is more acidic than HClO because of the resonance stabilization of its anion.
D
HClO4 is formed in the reaction between Cl2 and H2O.
Answer
HClO4 is more acidic than HClO because of the resonance stabilization of its anion.
Explanation
Solution
Acidic Nature : HClO4>HClO
H2O+Cl2→HCl+HOCl
 undefined
undefined
^
