Question
Question: The correct statement(s) about the oxoacids, \[HCl{O_4}\]and \[HClO\], is(are): This question has ...
The correct statement(s) about the oxoacids, HClO4and HClO, is(are):
This question has multiple correct options
A. The central atom in both HClO4 and HClO is sp3 hybridized
B. The conjugate base of HClO4 is weaker base thanH2O
C. HClO4 is formed in the reaction betweenCl2 and H2O
D. HClO4 is more stable than HClO because of the resonance stabilization of its anion.
Solution
We know that oxoacids are the acids that have oxygen atoms in it. They are different from hydrochloric acid. Oxoacid may contain sulphur, carbon and nitrogen elements from p-block.
Complete answer:
Option (A) The central atom in both HClO4and HClO is sp3 hybridized. The central Cl atom in HClO4 has 4 bonding domains and zero lone pairs of electrons. The oxygen atom in HClO has two bonding domains and two lone pairs of electrons.
Option (B) The conjugate base of HClO4 is weaker than water. This is because HClO4 is stronger acid than water. A stronger acid has weaker conjugate base and vice versa.
Option (D)HClO4 is more acidic than HClO because of the resonance stabilization of its anion. Greater is the extent of delocalisation, greater is the stabilisation of negative charge and higher is the acidity. In HClO4, negative charge is delocalised over four oxygen atoms.
Hence, the above statements (A), (B) and (D) are correct.
Now, let’s talk about the statement (C).
(C) HClO4 is formed in the reaction between Cl2 and H2O. This is not the correct statement. Instead, the following methods are used.
NaClO4+HCl→NaCl+HClO4
**Hence, the answer is (A)(B) and (D).
Note: **
Here the halogen can be any halogen from the halogen group of p-block.
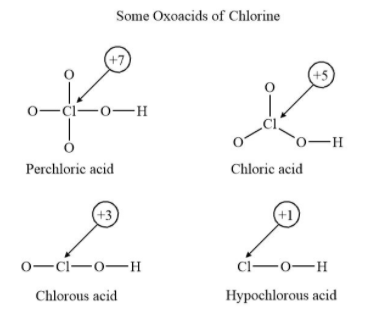
Here as shown in the figure the chlorine will have a different oxidation state. The change in oxidation state will be as per the bonding of the atoms with chlorine and hybridisation will be dependent on that.
