Question
Question: The correct statement about the synthesis of erythritol \( \left( {C{{\left( {C{H_2}OH} \right)}_4}}...
The correct statement about the synthesis of erythritol (C(CH2OH)4) used in the preparation of PETN is:
(A) The synthesis requires three aldol condensation and one Cannizzaro reaction
(B) Alpha hydrogens of ethanol and methanol are involved in the reaction
(C) The synthesis requires two aldol condensation and two Cannizzaro reaction
(D) The synthesis requires four aldol condensation between methanol and ethanol
Solution
Hint : PETN’s full Pentaerythritol tetranitrate. It is also known as PENT, PENTA, TEN or penthrite which is explosive material. PETN is a nitrate ester of pentaerythritol and has structural similarity with nitroglycerin. Generally, Penta refers to the five carbon atoms of the neopentane skeleton. The erythritol is the monomer of PETN.
Complete Step By Step Answer:
First, let’s observe the structure of erythritol which is having the chemical formula of (C(CH2OH)4) ;
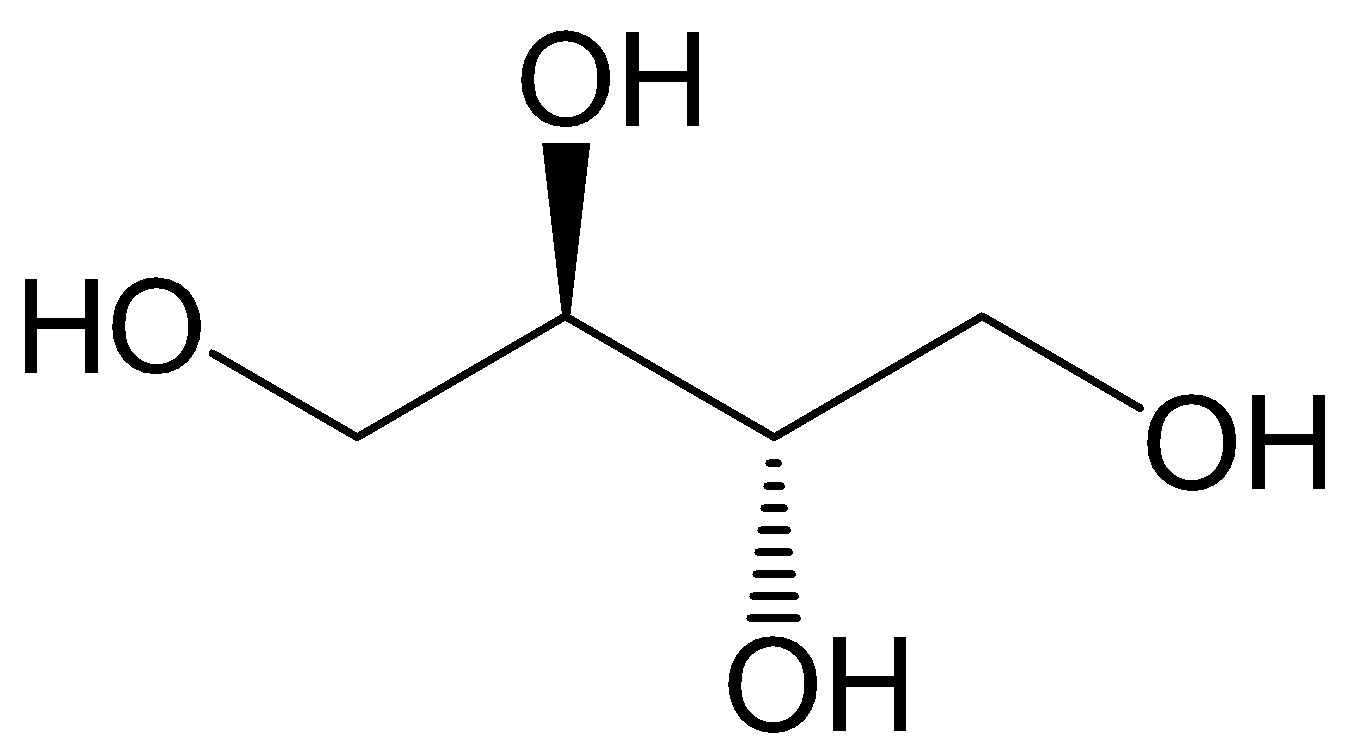
Let’s discuss the aldol condensation;
In aldol condensation, the formation of enolate ion takes place with the carbonyl compound having the α−hydrogen to form β−hydroxyketone or β−hydroxy aldehyde , which is followed by dehydration to give a conjugated enone. The aldol condensation plays a major role in organic synthesis, creating a path to form carbon-carbon bonds.
The reaction is given below;
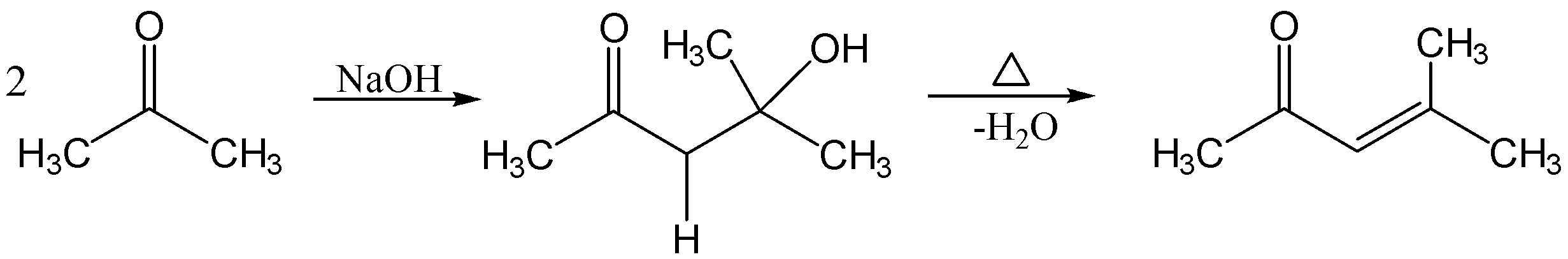
In the reaction, the two moles of acetone react with dilute NaOH which forms β−hydroxyketone followed by dehydration to form α,β−unsaturated ketone .
Now, let’s see the Cannizzaro reaction;
The Cannizzaro reaction is also known as a disproportionate reaction, in which the two moles of carbonyl compounds not having α−hydrogen will react with the concentrated base to form alcohol and acid.
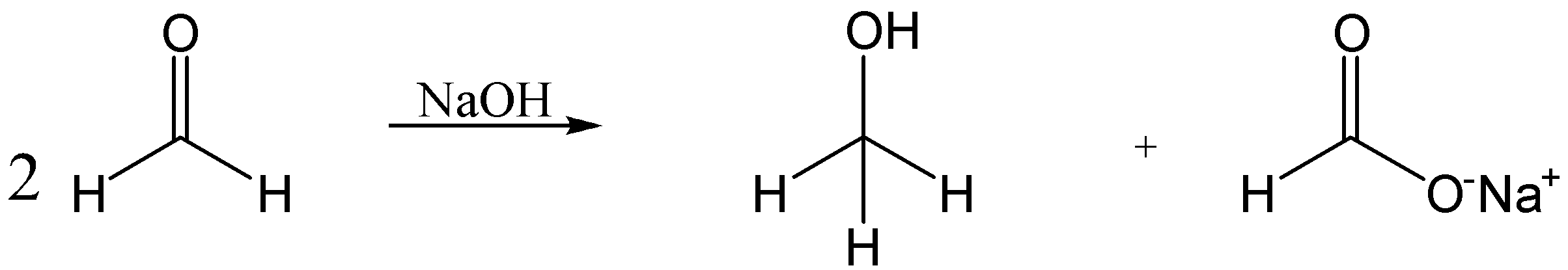
In this reaction, the two moles of formaldehyde react with conc. NaOH to form methanol and sodium formate.
If we observe, we need three times the aldol condensation so that we can obtain the non- α−hydrogen . Then we need to do Cannizzaro reaction to obtain our desired product erythritol (C(CH2OH)4) ;
The reaction is given below so that we can understand in a better way;
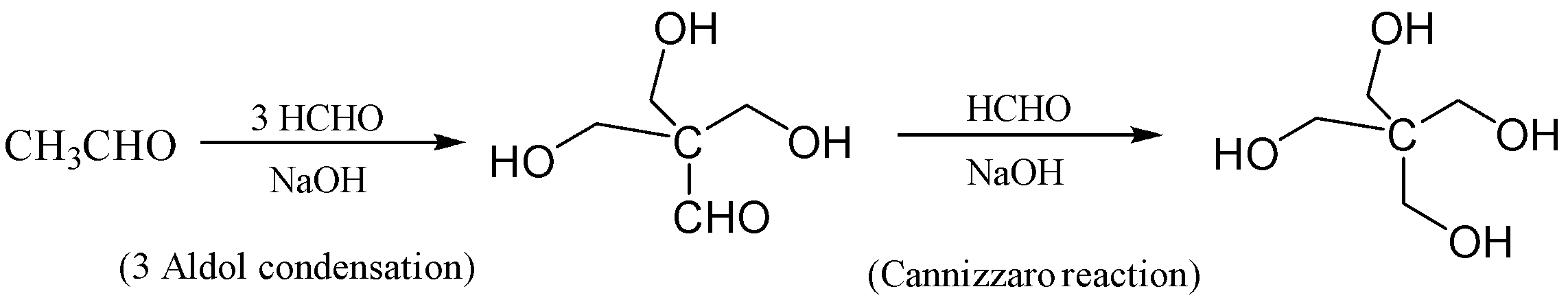
The correct answer is (A) The synthesis requires three aldol condensation and one Cannizzaro reaction
Note :
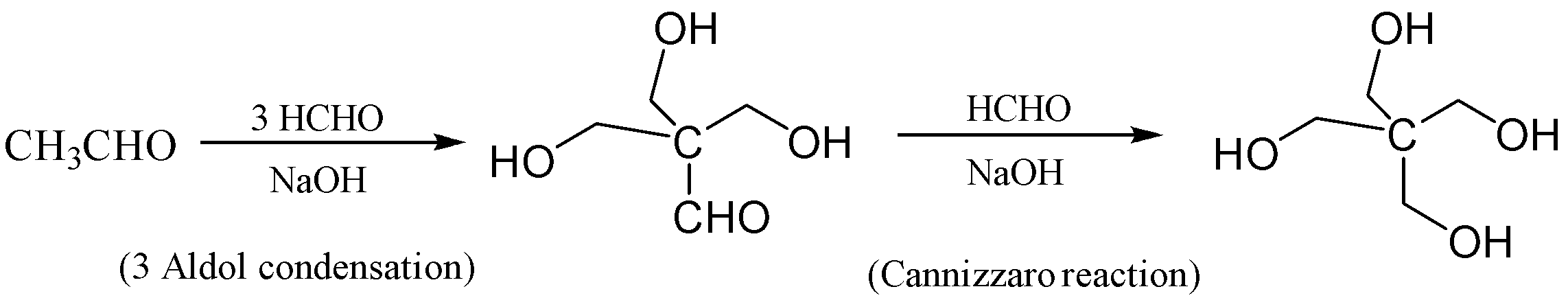
In this reaction, the ethanal is reacted with formaldehyde in the presence of excess sodium hydroxide which forms 3−hydroxy−2,2−bis(hydroxymethyl)propanal . This reaction occurs thrice which is aldol condensation. In the next step, the Cannizzaro Reaction occurs in which 3−hydroxy−2,2−bis(hydroxymethyl)propanal is reacted with formaldehyde we get 2,2−bis(hydroxymethyl)propane−1,3−diol which is also known as erythritol.
