Question
Question: The correct statement about the following molecule is: ...
The correct statement about the following molecule is:
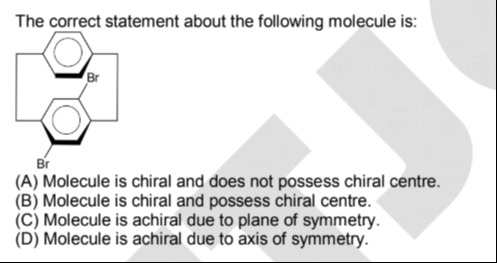
Molecule is chiral and does not possess chiral centre.
Molecule is chiral and possess chiral centre.
Molecule is achiral due to plane of symmetry.
Molecule is achiral due to axis of symmetry.
Molecule is chiral and does not possess chiral centre.
Solution
The molecule is a derivative of [2.2]paracyclophane. It consists of two benzene rings connected by two bridges. One bridge is a simple connection, and the other bridge connects the carbon atoms bearing the bromine substituents. The bromine atoms are attached to the para positions of the benzene rings. The stereochemistry of the bromine atoms is indicated by wedge and dash bonds, meaning they are on opposite sides relative to the plane of the benzene rings.
A molecule is chiral if it is not superimposable on its mirror image. Chirality is the absence of improper rotation axes (Sn), which include planes of symmetry (σ, S1) and centers of inversion (i, S2).
Let's examine the symmetry of the molecule. Due to the presence of the bromine atoms on opposite sides (one wedge, one dash), the molecule does not have a plane of symmetry that bisects it. For example, a plane passing through the centers of the benzene rings and perpendicular to the bridges would not be a plane of symmetry. Also, there is no center of inversion.
However, the molecule does possess a C2 axis of rotation. This axis passes through the midpoints of the two bridges. Rotation by 180 degrees around this axis superimposes the molecule onto itself. The presence of a proper rotation axis (like C2) does not make a molecule achiral.
Since the molecule lacks a plane of symmetry and a center of inversion, it is chiral.
Next, let's check for chiral centers. A chiral center is typically a tetrahedral carbon atom bonded to four different groups. In this molecule, the carbon atoms in the benzene rings are sp2 hybridized. The carbon atoms in the bridges are sp3 hybridized, but they are not bonded to four different groups in such a way as to be chiral centers. The carbon atoms attached to the bromine atoms are part of the benzene rings and are sp2 hybridized. Therefore, the molecule does not have any traditional chiral centers.
The chirality in this molecule arises from the overall arrangement of the atoms due to the restricted rotation of the benzene rings and the relative positions of the substituents. This is an example of axial or planar chirality, or more specifically, restricted rotation leading to atropisomerism in a cyclic system. In this case, it is a type of non-central chirality.
Therefore, the molecule is chiral and does not possess chiral centers.
